
|
A Thermodynamic Interpretation of History CHAPTER 10.A: The Progressive Bildung of Open Dissipative Structure 10.A.3: The Origin, Complexification, and Evolution of Life ACADEMY | previous section | Table of Content | next section | GALLERY |

|
A Thermodynamic Interpretation of History CHAPTER 10.A: The Progressive Bildung of Open Dissipative Structure 10.A.3: The Origin, Complexification, and Evolution of Life ACADEMY | previous section | Table of Content | next section | GALLERY |
(2004, 2005, 2006 by L. C. Chin. Incomplete manuscript.)
We can now give a brief narrative of the history of life on earth as illustration of the history of the formation of open dissipative structures -- a history of complexification. Our yard-stick for this complexification in terms of the simplified, three-stage version of Haeckel's (Plastide-Person-Corm) is adopted when the individuality of the open dissipative structure and the erasure of this individuality during its integration into a higher level of individuality are taken as the criteria of determination. If other criteria are adopted then the stages of this complexification will differ. John Maynard Smith and Eörs Szathmary, for example, in The Major Transitions in Evolution (1995 - 7), give different stages of this process of complexification when they view the increase of complexification of living organisms through time as dependent "on a small number of major transitions in the way in which genetic information is transmitted between generations" (p. 3). Without considering their schema until the end, the following will however make much use of the details of their marvelous reconstruction of the history of life on earth. Chris Colby's more plain and short narrative (at Talk-origin) will also serve as the contextual guide.
I. First stage in the progressive formation of open dissipative structures: cell or plastide. Colby gives a quick overview of this era:
|
Life evolved in the sea. It stayed there for the majority of the history of earth.
The first replicating molecules were most likely RNA. RNA is a nucleic acid similar to DNA. In laboratory studies it has been shown that some RNA sequences have catalytic capabilities. Most importantly, certain RNA sequences act as polymerases -- enzymes that form strands of RNA from its monomers. This process of self replication is the crucial step in the formation of life. This is called the RNA world hypothesis. The common ancestor of all life probably used RNA as its genetic material. This ancestor gave rise to three major lineages of life. These are: the prokaryotes ("ordinary" bacteria [Colby seems to mean eubacteria here]), archaebacteria (thermophilic, methanogenic and halophilic bacteria) and eukaryotes. Eukaryotes include protists (single celled organisms like amoebas and diatoms and a few multicellular forms such as kelp), fungi (including mushrooms and yeast), plants and animals. Eukaryotes and archaebacteria are the two most closely related of the three. The process of translation (making protein from the instructions on a messenger RNA template) is similar in these lineages, but the organization of the genome and transcription (making messenger RNA from a DNA template) is very different in prokaryotes than in eukaryotes and archaebacteria. Scientists interpret this to mean that the common ancestor was RNA based; it gave rise to two lineages that independently formed a DNA genome and hence independently evolved mechanisms to transcribe DNA into RNA. The first cells must have been anaerobic because there was no oxygen in the atmosphere. In addition, they were probably thermophilic ("heat-loving") and fermentative. Rocks as old as 3.5 billion years old have yielded prokaryotic fossils. Specifically, some rocks from Australia called the Warrawoona series give evidence of bacterial communities organized into structures called stromatolites. Fossils like these have subsequently been found all over the world. These mats of bacteria still form today in a few locales (for example, Shark Bay Australia). Bacteria are the only life forms found in the rocks for a long, long time --eukaryotes (protists) appear about 1.5 billion years ago and fungi-like things appear about 900 million years ago (0.9 billion years ago). Photosynthesis evolved around 3.4 billion years ago. Photosynthesis is a process that allows organisms to harness sunlight to manufacture sugar from simpler precursors. The first photosystem to evolve, PSI, uses light to convert carbon dioxide (CO2) and hydrogen sulfide (H2S) to glucose. This process releases sulfur as a waste product. About a billion years later, a second photosystem (PS) evolved, probably from a duplication of the first photosystem. Organisms with PSII use both photosystems in conjunction to convert carbon dioxide (CO2) and water (H2O) into glucose. This process releases oxygen as a waste product. Anoxygenic (or H2S) photosynthesis, using PSI, is seen in living purple and green bacteria. Oxygenic (or H2O) photosynthesis, using PSI and PSII, takes place in cyanobacteria. Cyanobacteria are closely related to and hence probably evolved from purple bacterial ancestors. Green bacteria are an outgroup. Since oxygenic bacteria are a lineage within a cluster of anoxygenic lineages, scientists infer that PSI evolved first. This also corroborates with geological evidence. Green plants and algae also use both photosystems. In these organisms, photosynthesis occurs in organelles (membrane bound structures within the cell) called chloroplasts. These organelles originated as free living bacteria related to the cyanobacteria that were engulfed by ur-eukaryotes and eventually entered into an endosymbiotic relationship. This endosymbiotic theory of eukaryotic organelles was championed by Lynn Margulis. Originally controversial, this theory is now accepted. One key line of evidence in support of this idea came when the DNA inside chloroplasts was sequenced -- the gene sequences were more similar to free-living cyanobacteria sequences than to sequences from the plants the chloroplasts resided in. After the advent of photosystem II, oxygen levels increased. Dissolved oxygen in the oceans increased as well as atmospheric oxygen. This is sometimes called the oxygen holocaust. Oxygen is a very good electron acceptor and can be very damaging to living organisms. Many bacteria are anaerobic and die almost immediately in the presence of oxygen. Other organisms, like animals, have special ways to avoid cellular damage due to this element (and in fact require it to live.) Initially, when oxygen began building up in the environment, it was neutralized by materials already present. Iron, which existed in high concentrations in the sea was oxidized and precipitated. Evidence of this can be seen in banded iron formations from this time, layers of iron deposited on the sea floor. As one geologist put it, "the world rusted." Eventually, it grew to high enough concentrations to be dangerous to living things. In response, many species went extinct, some continued (and still continue) to thrive in anaerobic microenvironments and several lineages independently evolved oxygen respiration. The purple bacteria evolved oxygen respiration by reversing the flow of molecules through their carbon fixing pathways and modifying their electron transport chains. Purple bacteria also enabled the eukaryotic lineage to become aerobic. Eukaryotic cells have membrane bound organelles called mitochondria that take care of respiration for the cell. These are endosymbionts like chloroplasts. Mitochondria formed this symbiotic relationship very early in eukaryotic history, all but a few groups of eukaryotic cells have mitochondria. Later, a few lineages picked up chloroplasts. Chloroplasts have multiple origins. Red algae picked up ur-chloroplasts from the cyanobacterial lineage. Green algae, the group plants evolved from, picked up different urchloroplasts from a prochlorophyte, a lineage closely related to cyanobacteria. |
I.A. The principles of complexification. Smith and Szathmary mention, "The idea of levels of organization, and hence of levels of selections, is central to [their] book." And so it is to ours, since complexification means precisely the ascendance to ever higher levels of organization which, in addition to conferring a common ancestry to all living organisms, characterizes the essence of evolution. But, as said, their additional concerns in the determination of the criteria for the yard-stick of the complexification of open dissipative structures called life result in their different spectrum of the "levels": "Not all major transitions, however, can be analyzed in this way. Perhaps the most important transition of all is that between organisms in which both genetic material and enzymes were made of RNA (the RNA world) and modern organisms in which the genetic material is DNA and enzymes are proteins -- a division of labour that requires that there be coding and translation [in addition to transcription]. A second transition, which also involves a change in the language whereby information is transmitted and in the physical medium that carries that language, is the origin of human speech. We accept this as being the decisive step in the origin of specifically human society. We will meet these two characteristics -- the division of labour and a change in language -- repeatedly." (Ibid., p. 12)
The division of labor means "an initially identical set of objects [single cells or plastide in a multicellular organism, i.e. Person, or Persons within a Corm]... becomes differentiated and functionally specialized... Why should selection favour such a division? The underlying principle can be illustrated by the evolution of dioecy from hermaphroditism... There are obvious advantages associated with hermaphroditism, so why are separate sexes so common? Let Rm and Rf be the reproductive success of males and females, respectively, and the reproductive success of a hermaphrodite be aRm as a male and bRf as a female. It is easy to show that dioecy will be favoured if a + b < 1. The main reason why this should be so is the existence of specialized organs, useful only to a male or to a female. Thus imagine a hermaphrodite red deer, investing half as much as a typical male in weapons and excess size, and half as much as a typical female in nourishing the young. In all probability, such a hermaphrodite would father less than half as many offspring as a typical male (a < 1/2), and would mother less than half as many offspring as a typical female (b < 1/2): that is, a + b < 1, and hermaphroditism would not pay. The stability of dioecy depends on the efficiency of specialized organs." (Ibid.) Since this thermodynamic interpretation of history has much concern with the gender question, this example of the division of labor, of differentiation and specialization, is especially relevant. Consider the list of the examples of differentiation-specialization Smith and Szathmary have provided, and look for their appearance in the narrative of the history of life.
|
The division of labor (differentiation and specialization) is the main mechanism by which higher levels of organization from plastidic through personic to cormic are progressively accessed by dissipative structures. If the formerly independent constituent may increase its viability or reproductive fitness by blending into a higher level of differentiation and specialization, the cost to it is precisely its independence, which is manifested most clearly in the fact that "entities that were capable of independent replication before the transition [to the higher level] can replicate only as part of a larger whole after it" (p. 6). The authors give the examples:
The first three (I - III) stages of complexification (division of labor) do not take the dissipative unit up to a new level of organization: the formation of chromosomes (below) constitutes the protocellular dissipative unit as a more integrated system; the symbiotic merger of one plastide with another (the mitochondrion specialized as the respirator and the host cell as the metabolizer) adds greater complexity to the already integrated plastidic dissipative unit; its differentiation into two sexes confers even greater complexity to the species. In the next two stages, however, the independence of the constituent is completely lost through the division of labor within the system.
Within a multicellular organism, the differentiation and specialization among individual cells that constitute their division of labor (there are, for example, 254 distinct cell types in a human Person) are produced not because the different cell types contain each different genes so as to differ from one another (all cells in the human Person have the identical genome), but because different genes of this same set of genome are active in different cell types ("epigenesis"). Within a cell only some of its genetic potentials are expressed while others repressed, in order for it to fit into its role within the whole organism. Within the Corm, the same situation holds. In a human society, individuals of different professions are expressing different parts of their common human potential and no one is expressing his or her full potential. Some (in fact, most) parts of the potentials of a person are repressed. Marx has called this "alienation".
As hinted at, Smith and Szathmary include a second determinant in the complexification of life in addition to the division of labor (differentiation and specialization): "Our thesis is that the increase [in complexity] has depended on a small number of major transitions in the way in which genetic information is transmitted between generations" (p. 3): change in "language". "Heredity means that like begets like: it requires some means whereby information can be transmitted. A crucial distinction... is between systems of 'limited heredity', in which only a few distinct states can be transmitted, and systems of 'unlimited heredity', capable of transmitting an indefinitely large number of messages" (p. 13). But a clear distinction must also be made between the vertical transmission of information (down to the next generations for the sake of reproduction) and its horizontal transmission (across the organism or society so that the constituents may bind together and harmonize in function with one another within the total system of the division of labor). The distinction between limited and unlimited heredity, obviously, is one within the vertical informational transmission, and this is the sole focus of the authors. The examples the authors give are:
We will in the following narrative run into every one of these significant advancements in the history of complexification from the biosphere to the noosphere.
I.B. The seeds in the prebiotic (geospheric) background for the bio-spheric formation of the plastide. Life (self-replicative open dissipative structures) only emerged within a "interaction sphere". Remember that this is the thermodynamic definition of life, its dual essential functions: metabolism and replication. Smith and Szathmary ask how "life" may have arisen with respect to these two aspects. "The simplest assumption is that, initially, metabolism consisted only of abiotic chemical reactions [autocatalysis, metabolism, dissipative cycle]... Such an abiotic metabolism supplied the monomers from which the first replicating polymers were synthesized, but those replicators did not themselves influence metabolism. Natural selection would act solely to select those replicators that multiplied best, in a chemical environment that they could not alter. This is a 'replicator-first', or 'gene-first', model of the origin of life. Only later did replicators evolve the ability to alter their own chemical environment. An alternative scenario is to dispense with specific replicating molecules, and to suppose that the metabolic system itself acquired a kind of heredity" (p. 18). The authors decide that "replicator-first" is the more plausible scenario. The task is then to explain the chemical environment where such replicators -- but also "the abiotic formation of sugars and amino acids, and of nucleotides or of some simpler molecules capable of base-pairing, and hence of template reproduction" -- could form. Lipids for the formation of membrane were only needed later, since the "individuals" of the ancestral interaction sphere (of "Progenotes") could not yet have cell wall, facilitating lateral gene transfer (p. 27; Goldenfeld, ibid.). The authors however reject the familiar idea of the "primitive soup" as such chemical environment, but favor "the more recent, and more promising, idea that the crucial chemical events took place between compounds bonded to a charged surface." This is because, first, "[c]hemical reactions will be both more frequent and more specific if the reacting molecules are moving on a surface, rather than in three dimensions". (Ibid.) (This is the "kinetic advantage of surface bonding": "many extant enzymatic reactions require the successful collision of three molecules. Such collisions are extremely unlikely in solution, but less so on a surface"; p. 33.) Second, such environment would favor over other reactions those which formed biological polymers (proteins, nucleic acids) requiring the removal of a molecule of water, and "this is difficult for molecules in solution in water, but easier on a surface" (p. 27). This is the thermodynamic advantage: when molecules combine in solution the resultant reduction in the freedom of their motion means the less likely entropy-decrease; "on a surface the elimination of a water molecule [in peptide formation] increases entropy, and so the reaction is more likely" (p. 32).
The charged surface on which the first metabolism should take place Smith and Szathmary identify as the pyritic. "The term pyritic means that the rock contains appreciable quantities of the brassy-colored mineral pyrite (iron sulfide, FeS2), also known as fool's gold" (J. William Schopf, "The Oldest Fossils and What They Mean", in Major Events in the History of Life, ed. J. William Schopf, 1992, p. 46). Because there was virtually no free oxygen in the prebiotic atmosphere (below), iron would not rust (into iron oxide) but would be "abundantly present in the oceans in a form, called ferrous, that is not found today because it would react immediately with atmospheric oxygen" (de Duve, Vital Dust, 1995, p. 16). Similarly pyritic conglomerates are virtually non-existent today, but their massive presence in the geologic record before 2 billion years ago points toward an atmosphere at the time that was devoid of free oxygen to oxidize them (Schopf, ibid., p. 47).
The question is of course whence came such chemical environment with the formation therein of the first bio-constituents. Christian de Duve summarizes the elements that in myriad molecular combinations make up the living world with the formula CHNOPS, "which stands for carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S)" (de Duve, ibid., p. 15). The first definitive scientific demonstration of the possibility of the abiotic (spontaneous) formation of life-essential molecules (amino acids and other organic molecules) of course is traced back to Stanley Miller's experiment in 1953.1 But that celebrated experiment (and what may be later called the Miller-Urey view) was based on the assumption of the prebiotic atmosphere as hydrogen-rich and composed of hydrogen (H2), methane (CH4), amnonia (NH3), and water vapor (H2O) (p. 16). This was a highly reducing atmosphere; but its specific chemical composition has since become suspect. "According to many experts, carbon was probably not present in combination with hydrogen (methane) but with oxygen (... CO2). Nitrogen most likely existed as molecular nitrogen (N2) or in one or more of its associations with oxygen, not as ammonia. Traces, at most, of molecular hydrogen were present." (Ibid.)2 In such atmosphere electric discharges (such as in thunderstorms) would no longer produce the bio-molecules in abundance (as their production becomes a thermodynamically uphill process rather than downhill in the original). Meanwhile a different trend arose favoring extraterrestrial origins of the bio-constituents. First of all, "[s]pectroscopic probing has revealed that the cosmic spaces are permeated by an extremely tenuous cloud of microscopic particles (interstellar dust) containing a number of potentially biogenic molecules, mostly highly reactive combinations of carbon, hydrogen, nitrogen, oxygen, and, sometimes, sulfur or silicon that would hardly remain intact under Earth conditions but could give rise to biologically significant compounds." (Ibid., p. 20; c.f. also, Stanley Miller, "The Prebiotic Synthesis of Organic Compounds as a Step toward the Origin of Life", in Major Events in the History of Life, ibid., p. 19) Then there were comets and meteorites: "Most stony meteorites do not contain much carbon, but there is a group of meteorites called carbonaceous chondrites that contain 0.5% to as much as 5% organic carbon." (Miller, ibid., p. 17) The most well-known of this type is of course the "Murchison" meteorite which fell on 28 September 1969 near Murchison, Australia, and contained 7 amino acids (glycine, alanine, valine, proline, glutamic acid, sacrosine, and a-aminoisobutyric acid) in the first report and 18 in the second (ibid.). The debate ensued then as to whether the first "seeds of life" came from outer space or originated on earth. "The Belgian-born American astrophysicist Armand Delsemme, from the University of Toledo, believes that virtually all the building blocks of life, as well as all the terrestrial water, were carried to the Earth by comets that contributed to the final accretion of our planet. According to Miller, on the other hand [c.f. also Miller, ibid.], the chemical precursors of life were formed mostly on Earth itself." (de Duve, ibid., p. 20) The extraterrestrial origin of the constituents of life is probably exaggerated, and the proposal of Smith and Szathmary that the first metabolism occurred on the surface of pyrite (FeS2) makes the nature of the atmosphere "largely irrelevant, since the crucial events took place close to hydrothermal vents in the deep sea." (S & S, p. 32) What the extraterrestrial abundance does teach is that the essential constituents of the biosphere are just an ordinary portion of the geosphere, and that under the right conditions (right temperature and right energy gradient) the geosphere (linear thermodynamic dissipation) could be expected to automatically transit to the biosphere (non-linear thermodynamic dissipation). The earth after its formation must have been coated with a layer of organic blanket consisting of various combinations of carbon, nitrogen, hydrogen, oxygen, sulfur, and which was ready to organize itself into more complex reaction cycles in certain regions (here, on the pyritic surface under the sea). But although the earth is 4.5 billion year old, the organic compounds could not assemble into the first autocatalytic cycle until 3.8 billion years ago when the intense bombardment of the earth by meteorites, which would have prevented any such assembly of metabolism, had stopped.
What is at issue here is the transition from the (dead) world of chemistry (of the geosphere) to the world of biology (biosphere) when these bio-constituents acquired autocatalytic and self-replicative capacity. Now was this first autocatalytic self-replicator made of nucleotides or enzymes? That is, after the first choice is made preferring "replicator-first", there is now the second of choices: "In existing organisms, nucleic acids and proteins mutually presume one another. The former, owning to their template activity, store the heritable information: the latter, by enzymatic activity, read and express this information... Which came first, nucleic acids or proteins?" Or did they coevolve? (S & S, p. 61) Because of Crick's "Central Dogma", "that information flows only from nucleic acids to proteins, never in the reverse direction" (de Duve, p. 22), it has been agreed that it must have been RNA which originally and undifferentiatedly assumed both the function of enzyme (proteinic) and that of replicator (nucleotidic). This means that it must have been the ancestral strands of RNA that were first formed on the pyritic surface. The specific steps which Smith and Szathmary posit led to the first "RNA organisms" are: formation on surface -> interaction/ cooperation between "strands" -> compartmentalization among them -> stochastic effect -> division of labor/ cooperation (i.e. complexification). (S & S, Ibid.) The RNA "organism" means that "[n]ucleic acids came first because they can perform both functions: they are replicable, and they can have enzymatic activity" (ibid.). "The affirmation [of the enzymaticity of RNA] gained a great boost in the early 1980s when two American investigators, Thomas Cech from the University of Colorado... and Sidney Altman from Yale University... found independently that certain RNA molecules were endowed with catalytic activity" (de Duve, p. 22). Cech subsequently dubbed the RNA enzyme "ribozyme" (ibid.). It is then that in 1986 Walter Gilbert postulated the RNA world in which "RNA molecules and cofactors [were] a sufficient set of enzymes to carry out all the chemical reactions necessary for the first cellular structures." (Cited by de Duve, ibid.) Contemporary examples of ribozymes -- these catalytic or autocatalytic RNA with enzymatic activity -- are the self-splicing RNA molecules in the Protozoan Tetrahymena. Recently, experiments, such as that by David Bartel and his colleagues at the Whitehead Institute for Biomedical Research (Nadia Halim, "Study Offers Insights into Evolutionary Origins of Life", 16 May 2001), have created "ribozymic organisms" from scratch in the laboratory. In this case, for example, the "ribozyme can use information from a template RNA to make a third, new RNA. It can do so with more than 95 percent accuracy, and most importantly, its ability is not restricted by the length or the exact sequence of letters in the original template. The ribozyme can extend an RNA strand, adding up to 14 nucleotides, or letters, to make up more than a complete turn of an RNA helix." The enzymaticity of RNA molecules should never cause surprise, for, just like proteins, "RNA often forms well-defined, flexible, three-dimensional structures, presenting various functional groups on its surface." (S & S, p. 62) In fact, the presence of nucleotide parts in many coenzymes today suggests that "they are remnants of ancient ribozymes ; and furthermore that the [nucleotide] cofactors are the fossil remains of an earlier complex ribozymic metabolism. On this argument, the reason why most of the few remaining ribozymes act on nucleic acid substrates [here we mean both components of the catalytic, enzymatic activity: substrate and the enzymic] is that the possibility of complementary base pairing makes them particularly effective for such reactions. At an earlier time, before the evolution of translation, ribozymes were involved in a much wider range of reactions: the remaining nucleotide cofactors are evidence of this, and provide clues to the nature of primitive ribo-organisms." (p. 64; emphasis added.) The identification of the first possible thing that can be called "organism" (an autocatalytic self-replicator) however is not the completion of the investigation into the origin of life (or the transition from geospheric chemistry to biospheric biology), since the spontaneous formation of RNA in the prebiotic chemical environment remains problematic. (p. 72) An alternative, "that primordial genes were made, not of RNA, but of clay", has been proposed, but dismissed. (p. 73 - 4) Conventional proposals (RNA-related) abound (p. 74 - 5), but no answer yet exists as to "how the primordial genetic material was formed", though all proposals "assume that the urgene was formed without a significant participation of protein" (p. 75).
I.C. The formation of the (genetic) information transmission system: from RNA to DNA translation. A puzzling question of the genetic code concerns why the "genetic alphabet" (nucleotides U, A, G, C, T) should consist of four letters (U and T being equivalent). Smith and Szathemary give the reason that "genetic alphabets made of two pairs [4 letters] [were] optimal in evolutionary terms; that is, organisms with two pairs would have had the highest fitness" (p. 75); or, "for ribo-organisms four bases were optimal." (p. 78) This is a matter of mathematics concerning the number of possible substrates on which they could act. The situation is not the same with enzymes: "an alphabet of 20 amino acids is not sufficient to ensure that a perfect enzyme can be produced for every substrate. It follows that an alphabet of 20 approaches, but does not quite reach, the size needed to generate a fully efficient set of enzymes." This is why in contemporary organisms "translationally modified amino acids sometimes play an important role in increasing catalytic efficiency" (p. 78).
The origin of translation and the genetic code is at the heart of the problem of how an RNA world can manage to transit into one "in which catalysis is performed by proteins, and nucleic acids specialize in the transmission of information" (p. 81), i.e. into the DNA world with translation of the nucleic acid genetic information to make catalytic (metabolic) and eventually structural proteins. The scenario proposed by Smith and Szathmary is that the association of particular codons with particular amino acids (the essence of translation and the genetic code, hence of the transition to the DNA world) originally "arose to serve quite a different function... [T]his is an example of a common feature of evolution: structures that today serve a complex function arose first to serve a simple one" (ibid.), and moreover: these contemporary structures came about through the twisting of structures originally doing something else into performing a new function. In the search for the origin of the code it is important to remember firstly that the pairing between codons and amino acids as seen today is not absolute "provided that some codons disappear transiently through directional mutational pressure, and then appear with an altered assignment" (p. 83); and secondly that "load minimization was a crucial factor in shaping the genetic code." (p. 85)
"Load minimization" refers to the two advantages which the coding of chemically similar amino acids by similar codons -- a visible feature of the contemporary genetic code -- may have: "First, genetic mutations, which usually change only one base in a codon, will have less damaging effects. Second... it would minimize the efforts of translational errors." (p. 84) Load minimization was achieved by originally having groups of amino acids encoded by groups of codons without sharp pairing of one codon with one amino acid. Something like arranged marriages en masse and without care of particular persons. "The evolution of the code was... like the coming into focus of an obscure picture." (p. 87) Differentiation from an original undifferentiatedness, that is.
This chemical connection of the genetic code points up the fact that there was code within the codons originating before the emergence of the translational machinery of tRNA and the ribosome system: "the first (5') position of the codon seems to be associated with biosynthetic kinship: codons of the amino acids belonging to the shikimate, pyruvate, aspartate and glutamate families tend to have U, G, A and C in this position, respectively. In contrast, polarity seems to be associated with the middle position." The first base of a codon also seems to be determinant of the amino acid size (purine [A and G] for small, pyrimidine [C, T, and U] for large), and the mid-base, of cloisteredness (purine for external, and pyrimidine for internal). (p. 88)
Lastly, note the redundancy of the contemporary code: e.g. Ser and Arg are coded for by 6 codons each, and "family boxes (the 4 codons for an amino acid) are quite common." (Ibid.) But Smith and Szathmary conclude that such redundancy in coding was determined by "factors that had nothing to do with amino acid functionality in proteins later in evolution" (p. 89), thus dismissing such suggestion as that "amino acids commonly used in proteins have more codons." (p. 88)
Now how did the three components of translation (excluding ATP) -- the "converter" (tRNA "adapter"), substrate (ribosome), enzyme-protein cofactors -- come about? Smith and Szathmary think that the function of the ancestral tRNA (a ribozyme) was to synthesize cofactor (an amino acid). This function eventually became "translation".
(1) The "RNA organisms" (so far, ribozymes) were using amino acids which acted as coenzymes for the ribozymes. These coenzymes (cofactors) consisted of an amino acid bonded to one or more nucleotide (oligonucleotide). "The amino acid would increase the chemical range and specificity of the ribozymes, and the nucleotides would constitute a 'handle'" connecting the cofactor to the ribozyme. (p. 89) Again, it was the physical shape of the bio-molecules that allowed "metabolism" (hence life) to happen.
(2) Chemical reasons dictated that the trinucleotide would make the ideal cofactor handle: ancestral codon arrangement.
(3) In the beginning, "a given cofactor could have been used by many different ribozymes." There was then no specific pairing between particular cofactor-synthesizing ribozymes (the future tRNAs) and particular amino acids as there is now between the particular tRNAs and particular amino acids. "The emergence of a specific assignment could have been aided by affinities between amino acids and nucleotides. It is known, for example, that hydrophobic amino acids in various solutions prefer the neighbourhood of A, the most hydrophobic base. Any such affinity could have been amplified by the active site of the cofactor-synthesizing ribozymes." (p. 90) This is part of the scenario of the gradual focusing of "mass coding" into "specific codings".
(4) The linking together of amino acids (cofactors): "Since nearly every function of ribozymes could have been fulfilled better by proteins, at least in principle, longer tracts of adjacent amino acids would have been formed on ribozymes. At this stage, the analogy to modern translating machinery is clear. The original nucleotide handles must have been transformed into ancient adaptors (primordial tRNA analogues)." (p. 91)
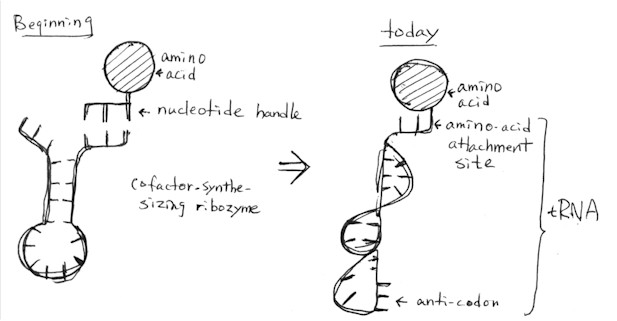
(5) Then the "adapter" (the ancestral tRNA: not sure whether Smith and Szathmary mean here the handle of the cofactor or the ribozyme synthesizing it or both fused together) became reusable and the ribozymes started differentiating their functions (division of labour): some became specialized as messenger and thereby lost their catalytic ability, others became integrated as subunits in functional ribonuceloprotein enzymes, and still others became the ancestors of ribosomal RNAs (the substrate for protein-synthesis; p. 91).
|----> messenger RNA
|
ribozyme ----- adapter-handle ----> tRNA
|
|----> ribosomal RNA
|
(6) The transition of the RNA to the DNA world would have however had to go through the transitory "ribonulcleoprotein world", where there was no precise pairing between RNA messenger's (tri-)bases and amino acids, and when "the original ribozyme served as a shrinking core or scaffold, onto which the novel protein structure assembled", and "parts of a ribozyme can be functionally replaced by a polypeptide." (p. 93) Of which the remnant, memory, today is that "some extant ribozymes come in two forms: alone or complemented by a protein." (Ibid.)
(7) Eventually, the fixed pairing between anti-codons and amino acids could have been generated by some sort of lock-and-key relationship (like that between enzymes and substrates) based on stereochemical affinity between the two sides, as demonstrated in in-vitro experimentations which today can produce "a whole array of different RNAs, each specific for a particular amino acid... [and also] different RNAs binding the same amino acids... Then one could test for the significant occurrence of anticodon-like structures in the selected molecules." This dispels the anxiety that the pairing "could hardly have arisen by chance alone" (p. 94).
The self-replicating nature of ribozymes (without the need for enzymes) avoided the problem of "error catastrophe" possible in this early era of the ambiguity of enzymes and pairing (of the translational process in general).3
Once the machinery of the DNA world (i.e. translation and the genetic code) had been established, the next step would be the liberation of metabolism from surface: the transition from its passive localization to an active process of membrane-generation and membrane-fission: i.e. the "first cell" (cellularization).
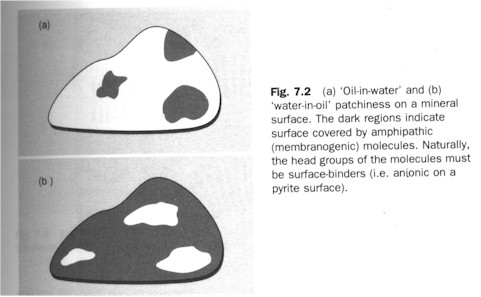 I.D. The boundary of life: membrane formation, the protocell (proto-plastide), and its metabolism. Recall that life (a cell or an organism) is an island of decreased entropy in a surrounding of increasing disorder. It thus must have a means to cordon itself off from the disordered (hence dangerous) environment: it needs a membrane. Smith and Szathmary note that although the constituents of lipids (fatty acids, glycerol, phosphate) were present in the pre-biotic soup, the possibility of the spontaneous formation of lipids therein was weak. But the presence of membranogenic, lipid-like material in the Murchison meteorite suggests that "some plausible abiotic pathway [to lipid-formation] must be available." (p. 101) Once lipids appeared on the pyrite surface, "they push water away, thus creating a more hydrophobic local environment. This makes the condensation reactions (leading, for example, to nucleic acids) more favorable, but also helps the formation of energetic anhydrids such as pyrophasphate and even ATP. This in turn helps the lipids to be equipped with strongly bonding pyrophosphate groups. Long-chain fatty acids could have formed by a variant of the archaic reductive citrate cycle on the pyrite surface." (Ibid.) The formation of lipids happened at the same time as the ribozymic metabolism (auto-catalysis) and replication were taking place. "Lipid formation results in the growth of lipid-covered surface area", that is, "oil-in-water" patchiness, which at some sites would reverse into a "water-in-oil" one. (Figure left.) "Further accumulation of lipids can result in semicellular structures by abstriction." The semicellular semi-organism was chemo-autotrophically autocatalytic, with ions provided by the pyritic surface; once the semicells became detached from the surface they would be genuine "protocells" (ibid.).
I.D. The boundary of life: membrane formation, the protocell (proto-plastide), and its metabolism. Recall that life (a cell or an organism) is an island of decreased entropy in a surrounding of increasing disorder. It thus must have a means to cordon itself off from the disordered (hence dangerous) environment: it needs a membrane. Smith and Szathmary note that although the constituents of lipids (fatty acids, glycerol, phosphate) were present in the pre-biotic soup, the possibility of the spontaneous formation of lipids therein was weak. But the presence of membranogenic, lipid-like material in the Murchison meteorite suggests that "some plausible abiotic pathway [to lipid-formation] must be available." (p. 101) Once lipids appeared on the pyrite surface, "they push water away, thus creating a more hydrophobic local environment. This makes the condensation reactions (leading, for example, to nucleic acids) more favorable, but also helps the formation of energetic anhydrids such as pyrophasphate and even ATP. This in turn helps the lipids to be equipped with strongly bonding pyrophosphate groups. Long-chain fatty acids could have formed by a variant of the archaic reductive citrate cycle on the pyrite surface." (Ibid.) The formation of lipids happened at the same time as the ribozymic metabolism (auto-catalysis) and replication were taking place. "Lipid formation results in the growth of lipid-covered surface area", that is, "oil-in-water" patchiness, which at some sites would reverse into a "water-in-oil" one. (Figure left.) "Further accumulation of lipids can result in semicellular structures by abstriction." The semicellular semi-organism was chemo-autotrophically autocatalytic, with ions provided by the pyritic surface; once the semicells became detached from the surface they would be genuine "protocells" (ibid.).
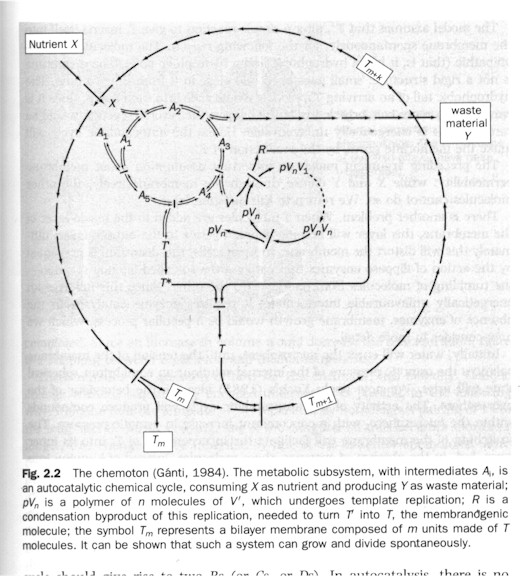
The protocell, as an autocatalytic cycle (like the theoretical chemoton: Figure above), must "generate the membranogenic molecules inside the system" in addition to metabolizing (dissipating). (p. 102) In its membranogenic and metabolic activity it must (1) synchronize autocatalytic and membrane growth, meaning: "surface area and volume increase at the same rate" (ibid.); (2) and divide spontaneously. The synchronization of autocatalysis and membrane growth leads to the spontaneous division because for the spherical shape "surface area and volume increase as the square and the cube of the radius", so that "the only feasible resolution is to divide into two when both total volume and total surface area are doubled, and two protocells of equal size [are] formed." (Ibid.) In any case, "fission may be explained by the fact that changes in surface area and volume drive the membrane through a series of quasi-equilibria, at the end of which the equilibrium is two equal-sized spheres." (p. 104) In other words it is thermodynamics in the end which gives rise to the primitive cell-division since the membranogenic autocatalytic cycle produces disequilibrium which can only resolve itself in fission.
Since the first metabolism must be autotrophic (S. & S. are emphatic about this, going against the traditional view of heterotrophism as the first) there is less of a problem of membrane transport as "only small molecules like H2S and CO2 must be taken up through the membrane" (p. 105). Thermodynamics means first of all that the concentration of materials inside the cell has to be lower than that outside. "This condition can be satisfied if they are consumed by metabolism; for example, monomers are converted into polymers, or metal ions are absorbed by nucleic acids or proteins" (p. 106). Secondly, some diffusion facilitators must be in place in the membrane -- obviously these could not yet be as complex as the modern day active transport proteins pumping at the expense of ATP (p. 105), but lipid molecules with -SH groups and fragments of RNA molecules linked to membranogenic molecules and serving as the carrier. "[O]rigin of protocells is unlikely without the prior evolution of ion carriers in some semicellular phase" (p. 106). What must have happened is that the as-yet surface-bound intermediary semi-cell metabolist with ion-carriers -- at first with ferrous, phosphate, and trace metal ions provided by the mineral grains of the pyritic surface -- then "spread laterally onto non-ferrous mineral grains", and finally onto ion-poor areas, where it definitely needed compounds facilitating the diffusion of ions inserted into its membrane (p. 105).
The protocell, membranogenically constituted from the semicell and thus liberated from the surface, is autotrophic aided by ribozymic fixation of CO2 -- still legacy of surface metabolism (p. 106 - 8). Again, the authors here are negating the formerly fashionable idea of heterotrophism as the ur-metabolism.
We need to recapitulate the surface autotrophism. If CO2 fixing autotrophism was the first autocatalytic cycle, then of the two extant CO2 fixations, the Calvin cycle [part of today's photosynthetic mechanism] and the reductive citric acid cycle, the latter is most likely of ancient origin, and its archaic form is posited as one "wherein the -SH groups coexist with the -OH groups and the carbonyls coexist with thio-derivatives", an especially plausible scenario because of "the surface-bonding properties of the carboxylate (-COO-) and the thio-carboxylate (-COS-) groups" (p. 106). This was "chemo-autotrophic, the energy for carbon-fixation... provided by the oxidation of FeS" (p. 107). But for an autocatalytic cycle to be a "metabolic subsystem of a (semi) cell, other products must be able to emerge from it as well. As Wächtershäuser suggested, an interesting concatenation of autocatalytic cycle is conceivable with intermediates of ever-longer carbon chains, finally leading to long-chain fatty acids, useful in lipid synthesis." (Ibid.) This answers the requirement of membranogenesis by autocatalytic cycle. "Several other side-reaction classes can be explained, leading to the following products: the amino acids aspartate and glutamate; the tetrapyrrol pathway leading, for example, to porphyrins; and the formation of phosphate ester from geminal thiol-phosphate groups." (Ibid.) As the origin of such (from the perspective of the first cell) "convenient" cycle S. & S. propose "a slow pyrite-pulled reductive carboxylation of carbon dioxide to oxalate (via thiocarbonate), which in turn may further be reduced and carboxylated to oxaloacetate." (Ibid.)
Now the constitution of the protocell means that while "the archaic cycle consists of surface-bonded intermediates", such evolution would require a "liberation of these molecules from the pyrite surface" (p. 107), that is, the transition from the semicell to the protocell was a "changeover from surface to cytosol metabolism" (p. 108), which possibly happened along the following lines:
S. & S. denied that ribozymes cannot have been sufficient for such cytosol metabolism, and that proteins (like iron-sulphur protein enzymes) were absolutely essential. Rather, they suggest that the protocell remained ribo-organismic, i.e. that RNA-based redox metabolism was possible, with the consequence that some crucial chemical processes today -- those in which RNA still plays a role, those that must be present at least in eubacteria (the oldest) but possibly in archaebacteria and eukaryotes as well, and those with no apparent selected functions in the extant biochemistry -- may have originated in this ribozymic metabolism. Notably "the involvement of adenine in extant biochemistry may be an ancestral relic" (p. 108) and before the evolution of translation and informed protein enzymes these protocellular ribo-organisms already developed DNA replication in place of RNA, synthesized tetrapyrrols (associated with the synthesization of chlorophyll) and terpenes (which could remove "the need for fatty acid biosynthesis in ribo-organisms"), and some of them furthermore had become photosynthetic ("photosynthesis, like DNA synthesis, is much simpler than translation, and confers a high selective advantage over chemosynthesis from the prebiotic pizza"; p. 109).
I.E. Toward the true plastide. The evolution of the protocell into a true cell then entails (1) the development of translation, as noted; (2) the evolution of enzymes: the as yet "unprogrammed polypeptides have weak and non-specific catalytic activity" (p. 110). This must have been the initial state: "a set of multifunctional enzymes" of "low efficiency, because the active sites of early enzymes [whether ribozymes or protein enzymes] could not be expected to provide a good and specific fit..." (Ibid.) The problem is how to evolve out of this undifferentiation or non-specialization and into the specificity of contemporary enzymes for precisely given reactions. "If every gene in the cell has a copy number of one", then "the system [of enzymes] is sitting in a mutational trap from which escape [through mutation] is difficult." (Ibid.) But when gene dosage is high, leading to "optimal dosage for each gene", such escape is possible: "given mutation, the end state of such a system was a set of highly specific enzymes. The crucial elements of the process are the duplication and divergence of genes, leading to an efficient division of metabolic labor between enzymes." (Ibid.)
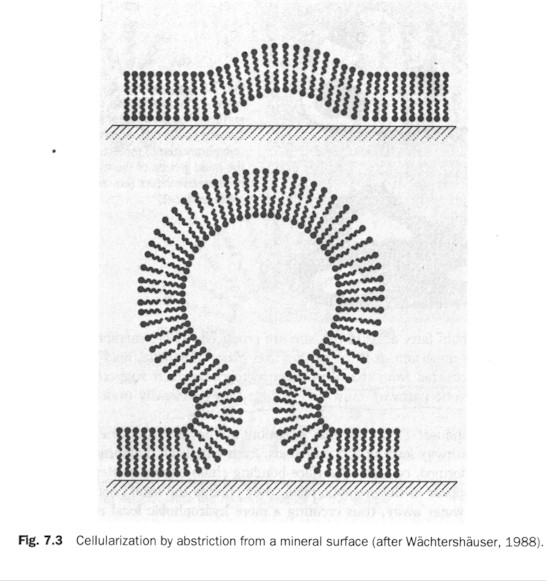
(3) The origin of the double membrane is most effectively gleaned from the study of negibacteria, "the most ancient extant group of life". The trick is the obcell which "by flattening, bending, and lip fusion" can form the "double membranes of the Gram-negative as opposed to single molecular bilayer" (p. 110. Figure below left). "Metabolic communication of the two membranes would have been possible by the newly formed Bayer's patches -- contemporary contact regions between the two membranes, where the cell wall is missing. The evolution of porins, preformed protein 'holes' in the outer membrane to let through all molecules with a weight less than about 600, would have happened during this transformation." (Ibid.) Against the approach that the second membrane of the Gram-negative evolved relatively late, S. & S. take "Cavalier-Smith's point of view... that the formation of the double-membrane was concomitant with that of the protocell itself" (p. 112). The scenario they argue for is that the semicells on the surface form into protocells with double membrane through semicellularization on, and abstriction from, the pyrite surface of a double membrane." (Ibid. Figure below right) Then subsequently the porin-like molecules are inserted into the outer membrane and the Bayer's patches appear to direct the traffic of membranogenic molecules coming from the semicell interior. "Those semicells possessing a more favorable membrane structure would spread to new surfaces through metabolic and membrane growth more rapidly than others lacking it." (Ibid.)
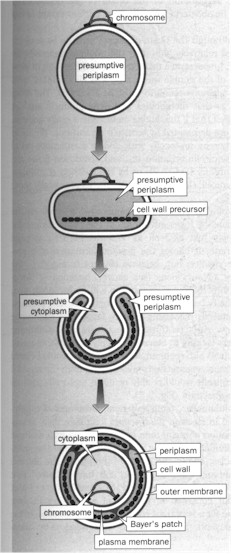
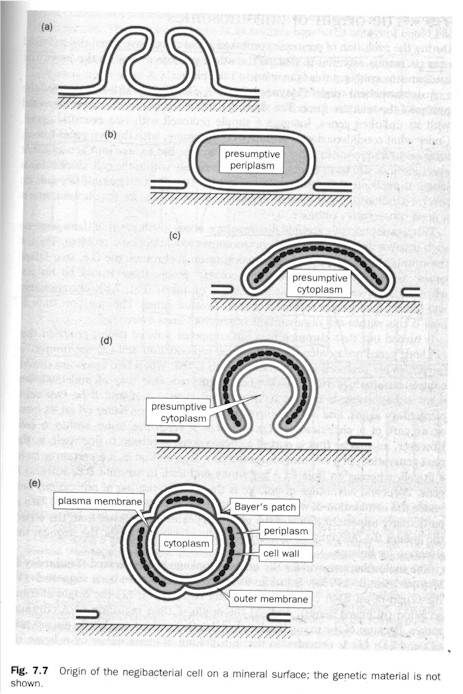
(4) In the protocell unlinked genes are linked up through chromosomes. S. & S. point out that although linked genes (chromosomes) take longer to replicate than isolated genes, they are still favored by selection [1] because linked genes have actually greater chance of "survival" (i.e. of re-appearance ) in the next generation since if that generation contains any one of the linked genes then the others linked to it would also be present; and [2] because the resultant synchronization of replication eliminates within-cell competition between different genes (p. 114). The change-over from RNA (single-stranded) to DNA (double-stranded) is related to the linkage of genes into chromosomes. "It might be thought that the main selective advantages of having a DNA genome is its increased stability (e.g. through the repair of single-stranded lesions) and decreased mutability (through the possibility of mismatch repair). However, all this would be possible in the case of double-stranded RNAs. Therefore, we believe that the increased chemical stability of deoxyribose and T (as opposed to ribose and U, respectively) was the primary selective force for the takeover by DNA" (p. 117).
I.F. A genealogy of the ancestral life. With the formation of true cell life in its proper sense has begun. How it diversifies into the myriad forms we witness in the history of life is the subject matter of systematics and captured in the diagram of "tree of life". Before continuing into the formation beyond the level of plastide we may briefly comment on the diversification of life that takes off from this true cell which has just successfully formed.
The traditional five-kingdom classification of all life on earth, first proposed by Robert Whittaker in 1959, has been supplanted since the 1990s by the three-domain system of Carl Woese, et al. The five kingdoms are: Monera comprising the single celled prokaryotes, Protista (roughly single celled eukaryotes), Plantae, Fungi, and Animalia, all three of which are multicelled eukaryotes. This system is still in use, with modifications, in Lynn Margulis et al, Five Kingdoms, today's standard book on biological taxonomy. With sequencing and comparison of ribosomal RNA, however, Woese has shown that a three-domain division underlies the traditional five kingdoms, with (eu-)Bacteria first diverging from Archae, from which then eukaryotes emerged later. The basic shape of the new tree of life is given below:
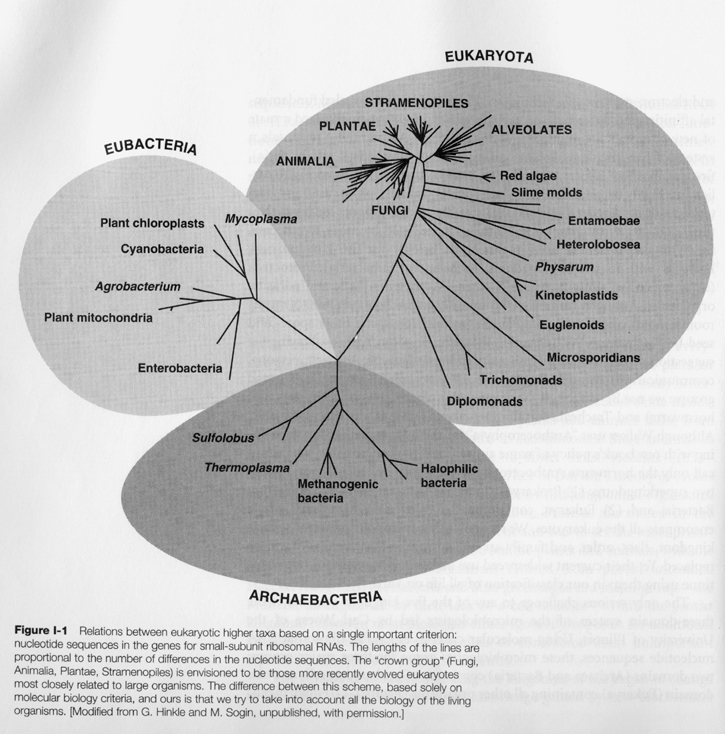
(Taken from Lynn Margulis, ibid., p. 8)
The new view has been implying, along with an early origin of life, also an early origin of all three domains, around 3.5 billion years ago. (Roger Lewin, Patterns in Evolution: The New Molecular View, p. 63)
More recently, the picture of the evolution of the earliest life has become more complex. The traditional construction of a tree of vertically descending lineages which, when traced backward in time, would converge at a root, has been confounded by the new realization of a large-scale horizontal transfer of genetic materials across the three domains during the earliest times.
| As Woese has written, "The ancestor cannot have been a particular organism, a single organismal lineage. It was communal, a loosely knit, diverse conglomeration of primitive cells that evolved as a unit, and it eventually developed to a stage where it broke into several distinct communities, which in their turn become the three primary lines of descent [bacteria, archaea, and eukaryotes]." In other words, early cells, each having relatively few genes, differed in many ways. By swapping genes freely, they shared various of their talents with their contemporaries. Eventually this collection of eclectic and changeable cells coalesced into the three basic domains known today. These domains remain recognizable because much (though by no means all) of the gene transfer that occurs these days goes on within domains. (Ford Doolittle, "Uprooting the Tree of Life", Scientific American, Feb. 2000) |
Something like the "Bacteria Interaction Sphere." The new picture is like this:
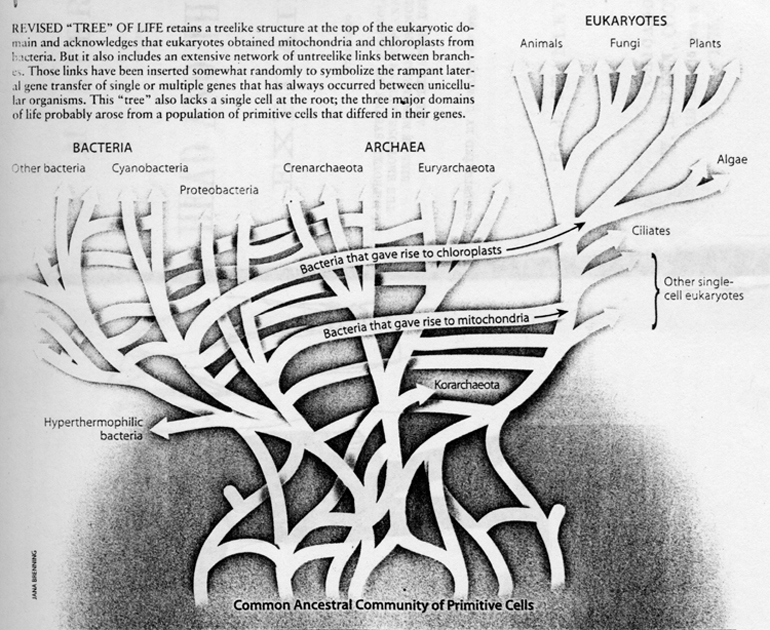
(Taken from Doolittle, ibid.)
One must notice here the same pattern we have noted before: From a vast ocean of simple forms only a small portion within (the eukaryotes) complexified into mutlicellularity and emerged to become the most dominant (at least most conspicuous). In the tree diagram, plants and animals, which dominated the activities on the surface of the earth, are mere two small twigs in the corner of Eukarya. It also says about the vast foundation of simplicity that is needed for the evolution therefrom of a thin strand of complexity.
More can be said about the universal ancestor of all life:
|
The range of ecological niches occupied by Bacteria and Archaea is both extremely broad and, to eukaryotic eyes, often bizarre. Modern thermophilic... bacteria live in hot springs and the vents of volcanoes, where temperatures are high and oxygen sometimes scarce; others live where levels of salt are high or pressure great. The distribution of these environments among the two domains gives a clue to the conditions under which life arose almost four billion years ago. Common to both domains are thermophilic organisms... Their broad presence implies that the earliest organisms, including the universal ancestor of the three primary groups, probably also occupied a harsh, high-temperature environment that sometimes exceeded the boiling point of water -- and originated there too. Modern eubacteria use oxygen to release energy from carbon compounds, converting it to water and carbon dioxide in the process. Four billion years ago, atmospheric oxygen was scarce, and the earliest organisms utilized other chemicals for this process, such as sulfur, which they converted to hydrogen sulfide. They used the energy released in this process to build organic molecules, using carbon dioxide as the basic raw material.
This... is fundamentally different from the classical view, which presumed that the first organism had been heterotrophic... that is, had relied on carbon compound such as glucose to build more complex molecules... (Roger Lewin, ibid., p. 60-1) |
Within this universal ancestor "pool of life" did one of the three domains emerge first or did they all emerge simultaneously? Evidence seems to point to eubacteria as the most ancient while eukaryote branched off from archaebacteria which itself branched off from eubacteria beforehand.
Footnotes:
1. There was once enthusiasm for the concept of spontaneous generation of life to explain the seemingly inexplicable appearance of life until it was discredited in 1880s by Louis Pasteur and with the development of cell theory and the germ plasm concept, which showed that a living cell could only come from a previously existing living cell. The history of the evolutionists' search for the origin of life ever since Darwin then had to pass through Haeckel's postulation of the unstructured protoplasm of "monera", Huxley's mistaken identification of Urschleim, and interests during the early 1900s in the suspended particles (colloidal suspensions) as anticipating the activity of life, before finally being set on the right track with Haldane and Oparin. (Peter J. Bowler, Evolution: the History of an Idea, rev. ed. 1989, p. 318 - 322.) Oparin's view in The Origin of Life (1936) was already modern and set the ground for Miller. "He followed Haldane in assuming [correctly] that the earth was originally a sterile planet with no free oxygen in its atmosphere. If oxygen had existed, it would have destroyed the chemicals necessary for the appearance of life. By treating oxygen as a product of living things, it was possible to uphold the claim that the evolution of life could only occur once in the planet's history." Oxygen is destructive of organic compounds because through its tendency to oxidate (snatching away the electrons of other atoms) "O 2 reacts relatively rapidly with organic compounds, especially in the presence of ultraviolet light [which would have abundantly penetrated into the prebiotic atmosphere due to the absence of the ozone layer, made up of O3]..." (Miller, ibid., p. 4) Hence while spontaneous generation of life was possible in the prebiotic atmosphere, once it had appeared life itself had changed the atmosphere so much that its spontaneous generation is no longer possible. "Oparin believed that the earth originally had been enveloped in a reducing atmosphere containing hydrocarbons and ammonia. Chemical reactions among these gases produced complex organic molecules that dissolved in the oceans to form a rich 'primordial soup.' Here the chemicals combined once again to form polymolecular open systems or coacervates, minute droplets with a definite structure, capable of absorbing materials from their surroundings. Natural selection now came into play, allowing these coacervates with more stable structures to survive at the expense of the less stable. The more successful structures became the 'prebionts', the intermediate stages before the appearance of true life in the form of the first cells. The last stage in the process was by far the most speculative part of the theory, however." (Bowler, p. 320)
2. As Campbell, Mitchell, and Reece summarize the contemporary opinion: "The first atmosphere [of Earth] was probably composed mostly of hot hydrogen gas (H2). Because the gravity of Earth was not strong enough to hold such small molecules, the H2 soon escaped into space. Volcanoes and other vents through the crust belched gases that formed a new atmosphere. Analyses of gases vented by modern volcanoes have led scientists to speculate that this second early atmosphere consisted mostly of water vapor (H2O), carbon monoxide (CO), carbon dioxide (CO2), nitrogen (N2), methane (CH4), and ammonia (NH3). The first seas were created by torrential rains that began when the planet had cooled enough for water in the atmosphere to condense. Not only was the atmosphere of the young Earth very different from the one we know today, but lightning, volcanic activity, and ultraviolet radiation [which the oxygenic waste by photosynthetic bacteria that oxygenated the atmosphere blocked through its formation of ozones, enabling aerobic multicellular life forms like us to prosper later] were much more intense. It was in such environment that life began." (Biology, 2nd ed., p. 321.)
3. "Suppose that, in any cell, translation errors lead to the production of malfunctioning proteins. This represents a loss of efficiency, but not a fatal one. Suppose, however, that some malfunctional proteins are themselves used in translation; for example, they are assignment catalysts. Then a single error in one round of protein synthesis could cause several errors in the next round. If so, there would be an exponential increase in the frequency of errors: an error catastrophe" (p. 94).
| ACADEMY | previous section | Table of Content | next section | GALLERY |