copyright © 2004 by L. C.
Appendix: Here is presented an exposition of organismic metabolism (whether cellular or multicellular) in order to both illustrate the analogy between religious rituals and biological metabolism and to contrast the primitive functional understanding of life-processes (metabolism) codified into religious rituals with the modern structural perspective of biology which invalidates any such "spiritual" understanding of life-processes.
(1) How an organism does work with the expenditure of energy. A. In biology the energy token is the ATP (Adenosine triphosphate) which is equivalent to the "sacrificial animal" on side of primitive religiousness. "ATP powers nearly all forms of cellular work, from the generation of light by fireflies to the movements of muscle cells that enable you to pedal a bicycle. Even as you read this page, nerve cells in your brain are using the chemical energy of ATP, energy your cells obtained earlier from food molecules" and deposited in the covalent bonds between the phosphate groups of the ATP. "When a cell uses chemical energy to perform work, it couples an exergonic reaction with an endergonic one." For instance, when cells want to break down the glucose molecules from food during digestion or move muscles during locomotion, the unstable covalent bond between the third and the second phosphate group is broken by hydrolysis, a phosphate thereby removed, ATP becoming ADP (Adenosine diphosphate), and energy exergonically released, and the protein molecule doing the work gains the energy endergonically by acquiring the removed phosphate, changing its physical shape in the process. "The added energy enables the molecule to perform work, here [by] changing back to its original shape [and losing the gained phosphate]. This kind of shape change is what helps a muscle cell to contract. The transfer of a phosphate group to a molecule is called phosphorylation, and most cellular work depends on ATP energizing other molecules by phosphorylating them." Phosphorylation is thus equivalent to the energization of the cosmos or the (atmospheric) god to do work, as when the Moabite king sacrifices his son (ATP) to "energize" ("phosphorylate") the cosmos-god. "Work can be sustained because ATP is a renewable resource that cells can regenerate. Figure C shows the ATP cycle. Energy released in exergonic reactions, such as glucose breakdown during cellular respiration, is used to regenerate ATP from ADP. In the process, a phosphate group is bonded to ADP, forming ATP by dehydration synthesis [an endergonic reaction]..." This re-phosphorylation of ADP into ATP is thus equivalent to the Intichiuma ritual for the regeneration of (what may be the sacrificial, or sacred) animals. "[A] working cell consumes and regenerates its entire pool of ATP about once each minute." (Campbell et al, p. 75)
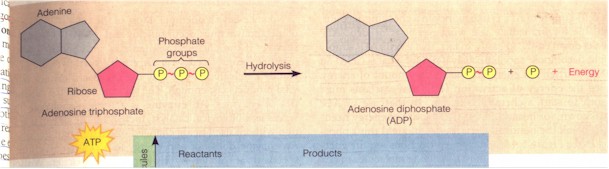
A. ATP structure and hydrolysis (Campbell, ibid.)
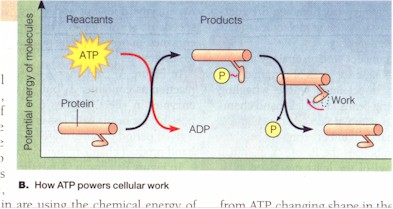 | 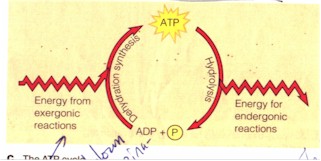
C. The ATP cycle (ibid.). |
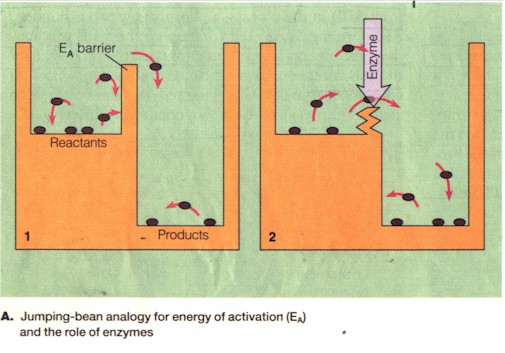 B. ATP and other vital molecules however do not breakdown spontaneously and become un-usable only because of the natural energy barrier, or the "amount of energy, called the energy of activation (EA), that reactants must absorb to start a chemical reaction. In the case of ATP breakdown, for instance, EA is the amount of energy needed to break the bond between the second and the third phosphate." In the left Figure, "[j]umping beans in the left-hand chamber of container 1 represent reactant molecules in a chemical reaction; those in the right-hand chamber represent product molecules. The partition symbolizes the EA of the chemical reaction." The left is higher than the right because the reactant is about to exergonically release energy. But to do so it "first has to have extra energy to make it over the partition. Jumping beans vary in how much energy they have. Those [most active]... jump the most often and the highest. At any particular instant, only a few... may jump high enough to clear the partition between the two chambers and become 'products'." This is the natural rate of breakdown or exergony: slow and low-yield. "Thus, it may take a very long time for a significant number of reactant beans to reach the right-hand chamber... [But] most of the essential reactions of metabolism must occur quickly and precisely for a cell to survive. If the chemical reactants of metabolism are like jumping beans faced with an energy barrier that cannot readily be cleared, a cell may die because it cannot make vital products fast enough. The solution lies in the capabilities of enzymes. An enzyme is a protein molecule that serves as a biological catalyst, increasing the rate of reaction without itself being changed into a different molecule.... it speeds up a reaction by lowering the EA barrier." Enzyme is thus the equivalent of the sacrificer, the priest or the shaman, etc. "Without enzymes, many metabolic reactions would occur too slowly to sustain life." (Ibid., p. 76)Similarly, without the sacerdotal personnel the cycle of the cosmos would continue too erratically to sustain the human social organism embedded therein.
B. ATP and other vital molecules however do not breakdown spontaneously and become un-usable only because of the natural energy barrier, or the "amount of energy, called the energy of activation (EA), that reactants must absorb to start a chemical reaction. In the case of ATP breakdown, for instance, EA is the amount of energy needed to break the bond between the second and the third phosphate." In the left Figure, "[j]umping beans in the left-hand chamber of container 1 represent reactant molecules in a chemical reaction; those in the right-hand chamber represent product molecules. The partition symbolizes the EA of the chemical reaction." The left is higher than the right because the reactant is about to exergonically release energy. But to do so it "first has to have extra energy to make it over the partition. Jumping beans vary in how much energy they have. Those [most active]... jump the most often and the highest. At any particular instant, only a few... may jump high enough to clear the partition between the two chambers and become 'products'." This is the natural rate of breakdown or exergony: slow and low-yield. "Thus, it may take a very long time for a significant number of reactant beans to reach the right-hand chamber... [But] most of the essential reactions of metabolism must occur quickly and precisely for a cell to survive. If the chemical reactants of metabolism are like jumping beans faced with an energy barrier that cannot readily be cleared, a cell may die because it cannot make vital products fast enough. The solution lies in the capabilities of enzymes. An enzyme is a protein molecule that serves as a biological catalyst, increasing the rate of reaction without itself being changed into a different molecule.... it speeds up a reaction by lowering the EA barrier." Enzyme is thus the equivalent of the sacrificer, the priest or the shaman, etc. "Without enzymes, many metabolic reactions would occur too slowly to sustain life." (Ibid., p. 76)Similarly, without the sacerdotal personnel the cycle of the cosmos would continue too erratically to sustain the human social organism embedded therein.
C. "Enzymes are very selective in the reactions they catalyze, and their selectivity determines which chemical processes occur in a cell at any particular time. As a protein, an enzyme has a unique 3-dimensional shape, and that shape determines the enzyme's specificity. A substance that an enzyme acts on -- a reactant... -- is called the enzyme's substrate." The substrate is here equivalent to the continuity, the spectrum, formed of the sacrificial victim and the cosmos. "Each enzyme recognizes only the specific substrate or substrates of the reaction it catalyzes. It therefore takes many different kinds of enzymes to catalyze all the reactions that occur in a cell." Hence the sacrificial religiousness of a culture has many different kinds of sacrifices and many different types of sacerdotal personnel -- each suited to a particular type of sacrifice -- in order to catalyze all the reactions needed to sustain the cosmo-social totality (harmony). To illustrate the working of the enzyme, the Figure shows the enzyme sucrase whose function is to catalyze the hydrolysis of its corresponding substrate table sugar (sucrose) to glucose and fructose: the substrate enters the active site of the enzyme and comes out in two. The king who sacrifices his son actually changes the very shape of the structure of the cosmos to include a victory for his kingdom.
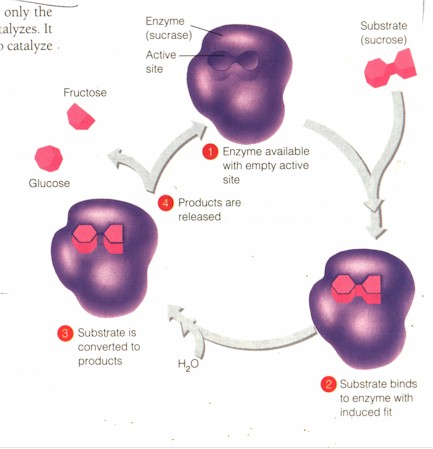
(Campbell, p. 77)
(2) Energy-harvesting via cellular respiration (rephosphorylation of ADP). We said that a used ATP, i.e. ADP, is regenerated during cellular respiration. Indeed, "harvesting energy [from food molecules ingested] is the fundamental function of cellular respiration." (The purpose of breathing, then, so to speak, is to convert food, as yet unusable, raw, into usable energy. Thus shall we compare the primitive, functional conception of breathing as "animation of the body" -- making live -- with the modern, structural understanding of it.) This replenishment of the energy currency of metabolism, which, as said, corresponds to the replenishment of the (sacrificial) animals, such as Intichiuma, is loosely speaking also the biological equivalent of the ritual firstlings sacrifices (thanksgiving) to ensure the future supply of the animals being hunted. "The chemical equation below summarizes cellular respiration as carried out by muscle cells, yeasts, and all other cells that use O2 in harvesting energy from the sugar glucose [the representative food molecule]... the starting (reactant) molecules glucose and O2 come apart, and their atoms regroup to form the products CO2 and H2O. In the process, glucose releases [when losing the hydrogen atoms] chemical-bonded energy, which the cell stores (or 'banks') in the chemical bonds of ATP." (Campbell, p. 90) In general all endergonic types of expiatory sacrifice performed after the reaping of certain results from the god-cosmos to repair the latter's exhaustion corresponds to such "banking". The imperfect efficiency of the "burning" (oxidation) of glucose during cellular respiration means that "a typical cell banks only about 40% of glucose's energy in ATP molecules... Most of the rest is converted to heat [i.e. wasted]." Compare this with the conversion by automobile engine of 25% of the energy in gasoline into the kinetic movement of the car. (p. 91)
|
C6H12O6 + 6O2 -------> 6CO2 + 6 H2O + ATP
|
Release of energy always involves destruction. In cellular respiration the energy in chemical bonds is harvested when glucose is dismantled and its electrons (with the protons they are attached to: hydrogen atoms) from the dissolved chemical bonds are shuttled "through a series of energy-releasing reactions... At each step in the sequence, electrons start out in a molecule [in the electron transport chain: below] where they have more energy and end up in one where they have less energy." (p. 92) The transfer of the electron from glucose to the series of molecules in the electron transport chain and finally to the oxygen inhaled represents the electron transfer, which is usually called redox reaction, i.e. reduction (gaining of electron, as by oxygen) plus oxidation (loss of electron, as by glucose or all the molecules that pass it along). This reaction, the shuttling of the hydrogen atom with its electron, is carried out by the enzyme dehydrogenase and the co-enzyme NAD+. "When the dehydrogenase strips two hydrogen atoms from an organic molecule, it is actually removing two protons... and two electrons... NAD+ picks up the two electrons and one [proton, conventionally designated as H+] and becomes NADH; it is thus a hydrogen carrier. The other [proton] goes into the surrounding solution in the cell." (p. 93) The NADH then delivers the electrons to the first of the electron carrier molecules of the electron transport chain embedded in the membrane of the mitochondrion inside the eukaryotic cell (In bacteria the electron transport chains are in the plasma). NADH then goes back to being NAD+. The redox reaction (oxidation of NADH and reduction of the electron carrier) "starts an electron cascade, in which electrons 'fall' down an energy 'hill' consisting of a series of electron carriers [the last being O2]. Each carrier is a different molecule; most are proteins... What keeps the electrons moving is that each carrier molecule has a greater affinity for electrons than its uphill neighbor", and O2 has the greatest. (p. 93) See figure below. The energy released from the downhill fall of the electron through the electron transport chain is used by some of the proteins built into the chain to transport the H+ ions (protons) across the membrane. The higher concentration of the H+ ions across the membrane is potential energy, as they tend to diffuse back to the less concentrated area (process of equilibrium, the second law of thermodynamics). As they do through the ATP synthase embedded also in the membrane of the mitochondrion (exergony) the ADP is re-phosphorylated (endergony). Most of the ATP in cells are produced by his "chemiosmotic phosphorylation" or "chemiosmosis". A minority is produced by substrate level phosphorylation, the transfer of a phosphate by an enzyme directly from an organic molecule to ADP (figure below).
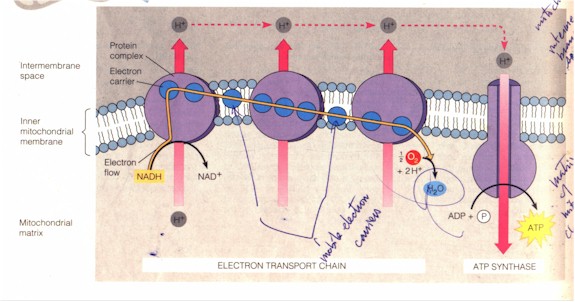
Complete view of the electron transport chain and ATP synthase
(Campbell et al, p. 100)
|
"Each of the oxygen atoms in O2 [as the final electron acceptor] combines with 2 electrons and with 2 H+ ions (from the surrounding solution) to form H2O, one of the final [waste] product of cellular respiration. Most of the carrier molecules reside in the 3 protein complexes (purple surface), which span the inner membrane of the mitochondrion... [Then] as redox occurs, the protein complexes use energy released from the electrons to actively transport H+ ions [i.e. protons] from one side of the membrane to the other... [i.e.] the mitochondron's intermembrane space... [Now] the H+ ions tend to be pushed back across the membrane into the matrix by the energy of the gradient [as a matter of the second law of thermodynamics]. However, the membrane is not very permeable... and [the protons] can only cross back by passing through a special protein port [of the ATP synthase]... As the [protons] move through the port, their flow drives the synthesis of ATP." (Ibid.)
|
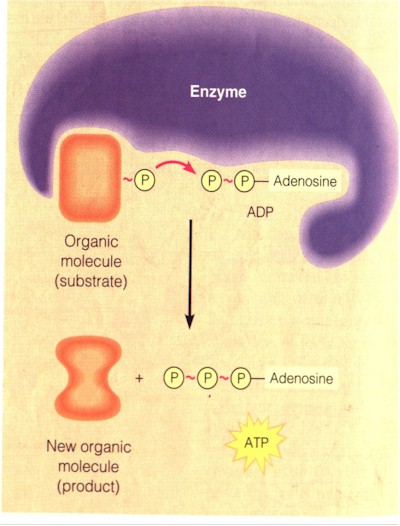
Substrate-level phosphorylation (Ibid., p. 94)
Before the manufacturing of ATP by ATP synthases in the mitochondrion membrane, however, two stages of cellular respiration occur first: glycolysis in the cytoplasmic fluid outside mitochondrion, breaking down glucose to two molecules of pyruvic acid and CO2, in the process generating 2 ATP; and the Krebs cycle within the mitochondrion, decomposing the pyruvic acids to CO2, generating also a few ATP. "The main function of glycolysis and the Krebs cycle, however, is to supply the third stage of respiration [electron transport chain] with electrons..." (p. 95) NAD+ during glycolysis and both NAD+ and FAD (flavin adenine dinucleotide) during Krebs cycle acquire electrons (get reduced) to become NADH and FADH2 and carry them to the electron transport chain. During the 9 steps of glycolysis (Figure below) -- an anaerobic energy-harvesting technique such as, e.g. utilized by yeast to produce the alcohol found in beer, and which occurs universally in all life forms, from the anaerobic bacteria to the aerobic humans, hence considered "similar to the process some of the first cells on Earth used to extract energy" (p. 96) -- the 6-carbon glucose molecule is split into two 3-carbon G3P during the preparatory (energy-consuming) phase, using up 2 ATP to phosphorylate the glucose and with the help of enzymes catalyzing the rearrangement and splitting of the chemical bonds; the 2 G3P are then during the energy-payoff phase each oxidized (deprived of hydrogen-electron) to yield 2 NADH and de-phosphorylated by enzymes to regenerate 4 ATP with 2 H2O as by-products. "The net gain of two ATP molecules [subtracting the two used in the first phase] from glycolysis accounts for only 5% of the energy that a cell can harvest from a glucose molecule. The two NADHs generated during steps 5 - 9 account for another 16%..." (p. 96)
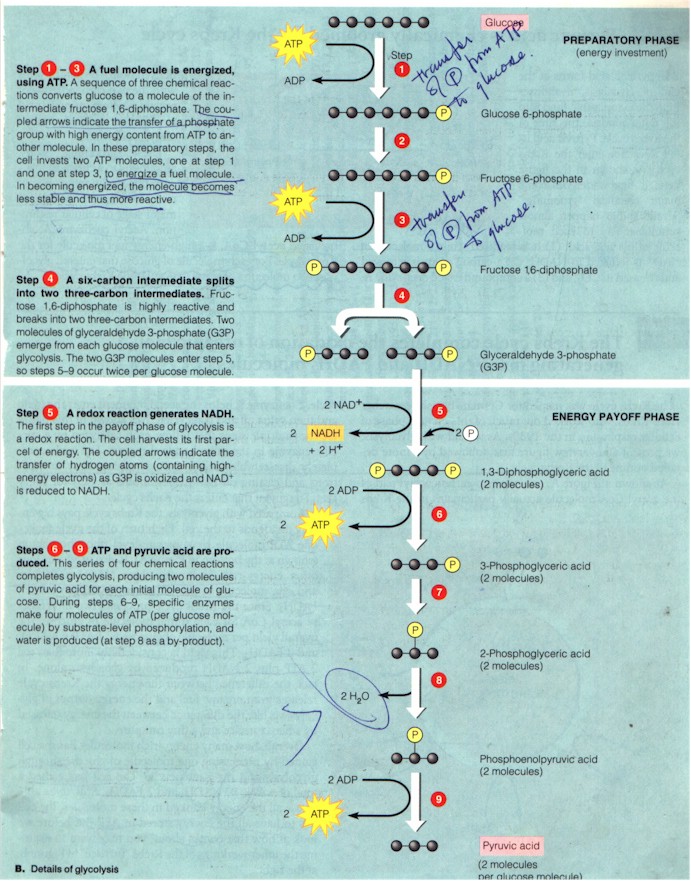
(From Campbell et al, p. 97)
Before the pyruvic acid enters the Krebs cycle, however, "it first undergoes chemical grooming." (1) It transfers one electron (hydrogen) to NAD+ (thence NADH); "(2) a carbon atom is [consequently] removed and released in CO2; and (3) a compound called coenzyme A, derived from a B vitamin, joins with the 2-carbon fragment remaining from pyruvic acid to form a molecule called acetyl coenzyme A." (p. 98; Figure below.)

Chemical grooming of pyruvic acid to Acetyl CoA (p. 98)
The Krebs cycle, worked out by the German-born British biochemist Sir Hans Adolf Krebs (1900 - 1981) in the 1930s, is also known as the tricarboxylic acid (TCA) cycle and as the citric acid cycle. "As shown in Figure [below], only the 2-carbon acetyl part of the acetyl CoA molecule actually participates in the Krebs cycle. Coenzyme A helps the acetyl fragment enter the cycle and then splits off and is recycled... [T]he multiple steps that follow [are] each catalyzed by a specific enzyme in the mitochondrial matrix. The cycle completely disassembles acetyl CoA, stripping away its electrons and casting off 2 carbon atoms as CO2 for every acetyl fragment that enters the Krebs cycle." (Ibid.)
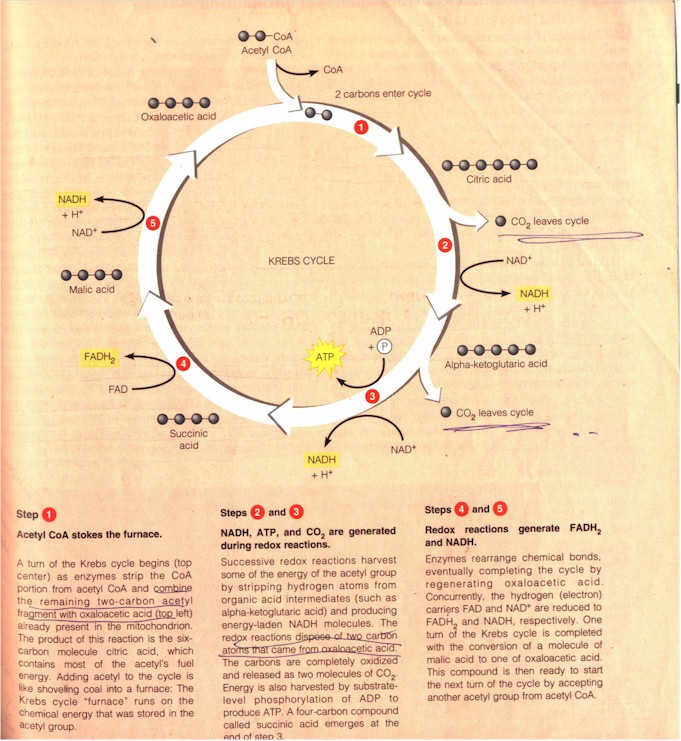
Details of the Krebs cycle (p. 99)
The overall yield by the Krebs cycle per each glucose molecule is: 2 x 1 = 2 ATP (produced in this cycle through substrate-level phosphorylation); 2 x 3 = 6 NADH; and 2 x 1 = 2 FADH2. Up til now glycolysis and the Krebs cycle together have produced (per each glucose molecule) 4 ATP, 10 NADH, and 2 FADH2. (p. 98)
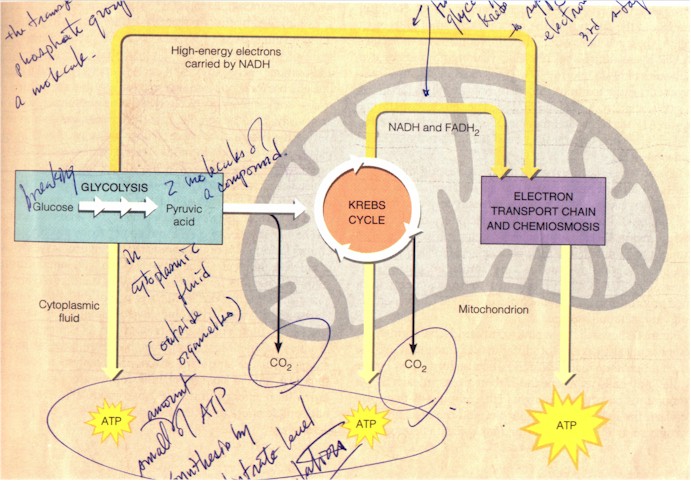
Complete view of all three stages of cellular respiration (p.95)
To sum up the energetic gain of cellular respiration performed on a single glucose molecule: glycolysis produces a net amount of 2 ATP, Krebs cycle 2 ATP, and approximately 2 ATP however are used up in shuttling NADH electrons from glycolysis out of the cytoplasmic fluid into the mitochondrion. If, as during maximally favorable conditions, "each NADH that transfers a pair of high-energy electrons from a food molecule to the electron transport chain contributes enough to the mitochondrion's H+ gradient to generate 3 ATP", and if "each FADH2 molecule yields 2 ATP" (p. 102), then chemiosmosis can produce 6 + 6 + 18 + 4 = 34 ATP. Since the electron transport chain depends on oxygen to operate, this means that an adequate supply of oxygen to the cell is essential for it to harvest energy and survive.
|-----------2 NADH (2 X 3 = 6)-----------------------------------|
| |
| |-----2 NADH (6)----->|-6 NADH(18)-2 FADH-2(4)--| |
| | | | |
glycolysis | | V V
gluc. -> 2 p.a. ---> 2 ACoA ---> Krebs cyc. E.T.C. & chemiosmosis
| | |
| | |
| | |
V V V
+ 2 ATP____- ca. 2 ATP___________+ 2 ATP______________________+ ca. 34 ATP
substrate-level substrate-level chemiosmotic
phosphorylation phosphorylation phosphorylation
Total: ca. 36 ATP
|
(Following Campbell et al, p. 102.)
(5) Metabolism in full view. While the primitives believe that the ingestion of food, as during a communion feast, is the reincarnation of the ancestral anima -- the same as the entrance of breath during birth -- the modern structural perspective of biology reveals that it is the intake of raw material, but not just for the conversion during cellular respiration to energy (or energy currency) that powers the movement of the body and its cellular constituents (on which we have concentrated), but also for the construction of these constituents themselves: biosynthesis. There are thus "two aspects of metabolism that are central to life : the energy-harvesting process of cellular respiration and the biosynthetic pathways used to construct all parts of the cell." (p. 105)
A. Returning to cellular respiration (energy-harvesting) to get the full view: "free glucose molecules are not common in our diet. In fact, we obtain most of our calories as fats, proteins, sucrose and other disaccharide sugars, and starch, a polysaccharide. You consume all these types of food molecules when you eat a bag of peanuts, for instance." (p. 104) When food is being digested, out of the four basic constituents of a biological organism three from the food molecules are being shuttled out to make ATP. (1) "A cell can funnel a wide range of polysaccharides and sugars into glycolysis [below]... For example, enzymes in our digestive tract hydrolyze starch to glucose, which is then broken down by glycolysis and the Krebs cycle. Similarly, glycogen, the polysaccharide stored in our liver and muscle cells, can be hydrolyzed to glucose to serve as fuel between meals." (p. 104) Thus can we get skinnier if without eating: the body "eats" itself. (2) "Proteins can be used for fuel, but first they must be digested to their constituent amino acids. Typically, a cell will use most of the amino acids to make its own proteins [i.e. biosynthesis], but enzymes will convert excess amino acids to other organic compounds. During the conversion, the amino groups, which can be toxic, are stripped off and disposed of in urine. The other parts of the amino acid molecules are usually converted to pyruvic acid, acetyl CoA, or one of the organic acids in the Krebs cycle, and their energy is then harvested by cellular respiration." (Ibid.) (3) "Fats make excellent cellular fuel because they contain many hydrogen atoms and thus many energy-rich electrons... [T]he cell first hydrolyzes fats to glycerol and fatty acids. It then converts the glycerol to glyceraldehyde 3-phosphate (G3P), one of the intermediates in glycolysis. The fatty acids are changed into acetyl CoA and then enter the Krebs cycle. Processed this way, a gram of fat yields more than twice as much ATP as a gram of starch." (Ibid.)
Food (e.g. peanuts)
|
|-----------------------------|------------------------------|
V V V
polysaccharides |- fats --| proteins
| | | |
V V V V
sugars |------------ glycerol fatty acids amino acids
| | | |
| | | |
| | |---------------|---|----------------|------> amino
| | | | | | group
| | | | | | (urine)
V V V V V V
glucose ---> G3P ---> pyruvic acid ---> Acetyl CoA -----> Krebs cycle --> ETC
(GLYCOLYSIS) | chemiosmosis
| | |
| | |
|-------------> ATP <----------------------------|----------|
|
Pathways of energy-harvesting or cellular respiration (Following Campbell et al, p. 104.)
B. "Because cells need matter as well as energy, not all food molecules are destined to be oxidized as fuel for making ATP. Food also provides the raw materials a cell uses for biosynthesis, to make its own molecules for repair and growth. Cells obtain some raw materials directly from food. Amino acids, for instance, can be incorporated without further change into an organism's own proteins. However, the body also needs certain molecules that are not present in the same form in food. Our cells make these molecules using some of the compounds of glycolysis and the Krebs cycle." (p. 105) The biosynthetic pathways (figure below) by which cells make the three of the four classes of cellular macromolecules are almost the reversal of those of energy-harvesting with the expenditure of ATP, with one crucial difference: "Although some of the key components are the same in the two processes [of glycolysis and glucose synthesis], the pathway of glucose synthesis from pyruvic acid is not the exact opposite of glycolysis." (Ibid.)
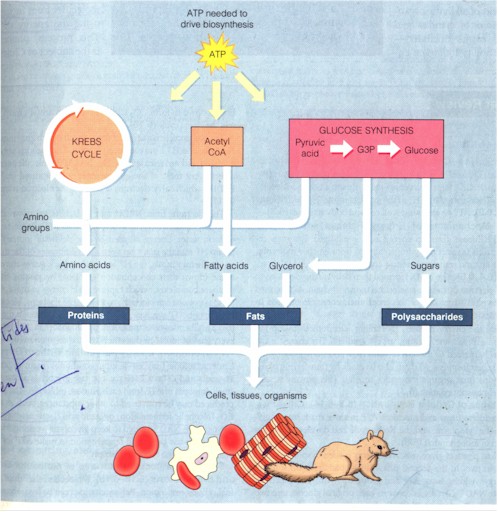
Biosynthesis (Campbell et al, p. 105)




 B. ATP and other vital molecules however do not breakdown spontaneously and become un-usable only because of the natural energy barrier, or the "amount of energy, called the energy of activation (EA), that reactants must absorb to start a chemical reaction. In the case of ATP breakdown, for instance, EA is the amount of energy needed to break the bond between the second and the third phosphate." In the left Figure, "[j]umping beans in the left-hand chamber of container 1 represent reactant molecules in a chemical reaction; those in the right-hand chamber represent product molecules. The partition symbolizes the EA of the chemical reaction." The left is higher than the right because the reactant is about to exergonically release energy. But to do so it "first has to have extra energy to make it over the partition. Jumping beans vary in how much energy they have. Those [most active]... jump the most often and the highest. At any particular instant, only a few... may jump high enough to clear the partition between the two chambers and become 'products'." This is the natural rate of breakdown or exergony: slow and low-yield. "Thus, it may take a very long time for a significant number of reactant beans to reach the right-hand chamber... [But] most of the essential reactions of metabolism must occur quickly and precisely for a cell to survive. If the chemical reactants of metabolism are like jumping beans faced with an energy barrier that cannot readily be cleared, a cell may die because it cannot make vital products fast enough. The solution lies in the capabilities of enzymes. An enzyme is a protein molecule that serves as a biological catalyst, increasing the rate of reaction without itself being changed into a different molecule.... it speeds up a reaction by lowering the EA barrier." Enzyme is thus the equivalent of the sacrificer, the priest or the shaman, etc. "Without enzymes, many metabolic reactions would occur too slowly to sustain life." (Ibid., p. 76)Similarly, without the sacerdotal personnel the cycle of the cosmos would continue too erratically to sustain the human social organism embedded therein.
B. ATP and other vital molecules however do not breakdown spontaneously and become un-usable only because of the natural energy barrier, or the "amount of energy, called the energy of activation (EA), that reactants must absorb to start a chemical reaction. In the case of ATP breakdown, for instance, EA is the amount of energy needed to break the bond between the second and the third phosphate." In the left Figure, "[j]umping beans in the left-hand chamber of container 1 represent reactant molecules in a chemical reaction; those in the right-hand chamber represent product molecules. The partition symbolizes the EA of the chemical reaction." The left is higher than the right because the reactant is about to exergonically release energy. But to do so it "first has to have extra energy to make it over the partition. Jumping beans vary in how much energy they have. Those [most active]... jump the most often and the highest. At any particular instant, only a few... may jump high enough to clear the partition between the two chambers and become 'products'." This is the natural rate of breakdown or exergony: slow and low-yield. "Thus, it may take a very long time for a significant number of reactant beans to reach the right-hand chamber... [But] most of the essential reactions of metabolism must occur quickly and precisely for a cell to survive. If the chemical reactants of metabolism are like jumping beans faced with an energy barrier that cannot readily be cleared, a cell may die because it cannot make vital products fast enough. The solution lies in the capabilities of enzymes. An enzyme is a protein molecule that serves as a biological catalyst, increasing the rate of reaction without itself being changed into a different molecule.... it speeds up a reaction by lowering the EA barrier." Enzyme is thus the equivalent of the sacrificer, the priest or the shaman, etc. "Without enzymes, many metabolic reactions would occur too slowly to sustain life." (Ibid., p. 76)Similarly, without the sacerdotal personnel the cycle of the cosmos would continue too erratically to sustain the human social organism embedded therein.







