ACADEMY & GALLERY
2003, 2005, 2006 Lawrence C. Chin. This page is ideally displayed in Internet Explorer. In some browsers, like Firefox and Safari, the mathematical equations on this page often do not display correctly. In such case, you may consult the PDF version of this webpage. The "Old Quantum Theory" Quantum theory evolved from a "old quantum theory" to the completed form of "quantum mechanics", which then further evolved into "quantum field theory", which is now the limit of the structural understanding of the geosphere. The emergence of quantum theory opens up a new chapter in the evolution (history) of science which we have modelled as something like the progressive marching-out of the Platonic cave. By the late nineteenth century, the structural perspective had come to realize that all reality consisted of tiny physical indivisibles called "atoms". But what did an atom look like? The "old quantum theory" resulted from the attempt to explain the structure of this atom. In successfully explaining the atomic structure, it unified the tradition descending from classical mechanics (the quantitative description of macroscopic [visible] objects in motion) with electrodynamics (in another way than did special relativity) and the other tradition crystallized around chemistry (in the beginning via the medium or methodology of thermodynamics). The old quantum theory therefore served as the first step in (finally?) laying bare the constitutive structure of the geosphere.1 The atomic or molecular material conception of reality (of the structural perspective) was most manifest in the Scottish physicist James Clerk Maxwell's statistical quantitative description of gases (his kinetic theory of gases) in 1859. Here the gaseous volume of any element (or compound) was pictured as billions of the atoms (or molecules) of this element (or compound) moving at random, colliding with each other and with the walls of the container, and with a velocity (and therefore kinetic energy) determined by the temperature of the gas: the heat added to the system was transformed into the kinetic energy of the atoms or molecules which then moved faster and collided with each other and with the walls more often (the relationship between the kinetic energy and the momentum of the molecule is today defined as E = p2/2m) -- gaseousness being the effect of this underlying structure. Since even a small quantity of gas, say, one mole, contained about 6 x 1023 molecules, a quantitative description of their movement (of the whole "system") could only be a statistical average, which Maxwell produced using Newtonian mechanics, showing that temperature was a measure of the microscopic mean (average) squared velocity of the molecules (T = v2). A curve called the "Maxwell Distribution" was then produced, giving information about the billions and billions of molecules (i.e. about the whole system), even though the motion of an individual molecule could not be calculated. The conformity of the majority of the atom-molecules to the average calculated denoted that the system was in equilibrium. Improving on this kinetic theory of Maxwell, Ludwig Boltzmann produced a statistical mechanics that could quantitatively described any collection of entities which have mutually independent freedom of movement and interact with each other randomly; for this he formalized a theorem of the equipartition of energy, that when the system reached equilibrium (maximal entropy) the energy would be shared equally among all degrees of freedom by all elements of the system. 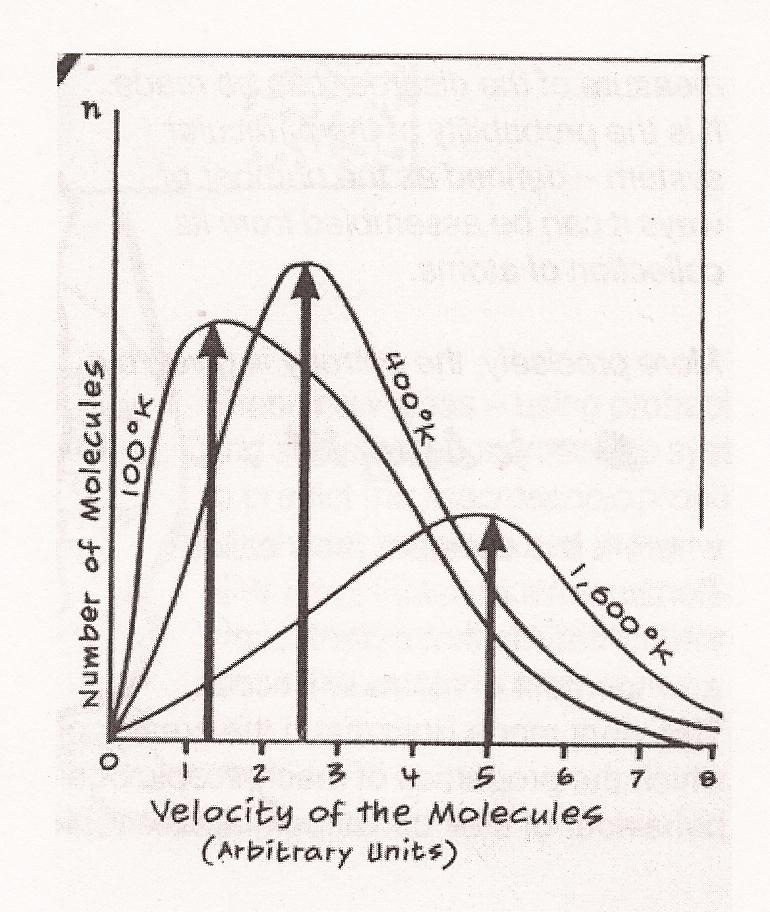 Maxwell Distribution
where c was the speed of light, T the temperature, l the wavelength and kB = 1.38 x 10 -23 the Boltzmann constant (Vacarro). This formula, although it predicted well the low frequency part of the experiment, diverged radically with the high frequency part. The problem with the Raleigh-Jeans approach was that it was applying the statistical method to electromagnetic waves by analogy with Maxwell's equipartition of energy among gas particles. In other words, the Raleigh-Jeans solution was in the classical manner, defining the quantity I(l, T)dl "as the power radiated (per unit area) in the wavelength interval dl". This would predict that "as the wavelength decreases, the power [I(l, T)] should tend toward infinity" (ibid.). That is, it predicted an ultraviolet catastrophe, which meant that anyone looking into the cavity of the black body, or anyone sitting in front of a fire place, should be burnt dead. In fact, "we would be bathed in intense ultraviolet light which would annihilate all life!" (Ibid.) Since this was not happening, this eidos -- together with that other of Wien's -- were clearly not the right ones. 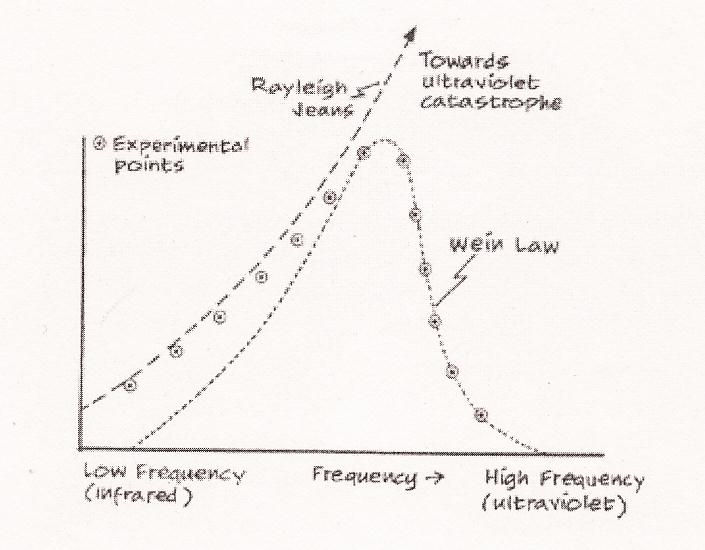 Quantum theory properly began when in 1900 Max Planck attempted to explain away this ultraviolet catastrophe. In other words, he was trying to find the right eidos. Since at the time nothing was known about the atoms, in manner similar to that in which Maxwell conceived of gas as consisting of discrete molecules moving at random, Planck thought of the walls of the cavity of the black body as consisting of a collection of "electric oscillators" vibrating back and forth under thermal agitation: as the temperature was raised the oscillator were caused to vibrate faster and faster so that the average frequency of radiation increased as well (but all possible frequencies were always present). The electromagnetic theory, although it could tell about (give quantitative description -- eidos -- of) the emission, absorption, and propagation of the radiation, could not as yet say anything about the distribution of energy during equilibrium condition among the waves bouncing about inside the cavity. This was a thermodynamic problem. Abandoning the formula of Wien (which was wrong for the low frequency, infrared area), and trying to relate the average energy of the oscillators to their entropy (the state of equilibrium or uniform distribution), Planck obtained a formula that at last agreed with all of the experimental data together, at high and low frequencies (it seemed to be the right eidos):
where E is energy, f frequency, T temperature, exp the natural exponent, and c1 and c2 constants chosen to make the equation fit the experiments. This Planck presented at the physics seminar at the University of Berlin, 19 Oct. 1900. Trying to understand the physical meaning of his equation, Planck applied Boltzman's formulation of entropy (S = kLogw, where k = Boltzman's constant and w = the probability that a particular arrangement of atoms will occur) and divided the energy of the oscillators into arbitrarily small but finite chunks. The total energy E of the system was then Ne, i.e. the total number (N: an integer) of e (an arbitrarily small amount of energy). e should eventually be reduced to an infinitesimal amount, following the classical procedure. Planck tried to correlate the energy unit (e) proportionally with the oscillator frequencies (f), and thus e = hf, and obtained the total energy of the system, E = Nhf. h, the chosen constant proportionality between energy level and frequency, in the classical manner, should decrease eventually to 0. But now, as Planck discovered, if h was required to decrease to 0, then the validity of his formula would be destroyed; if not, he would obtain the formula again. e = hf therefore could not, as in the classical picture of reality as continuous, be indefinitely decreased to 0, but must be a finite amount; thus h -- the Planck's constant was thus born -- was not 0. In other words there was a smallest possible amount of energy ordained by the universe and it was not possible for an oscillator to absorb and emit energy in a continuous range, but only discontinuously, in multiples of the smallest, indivisible unit of energy e = hf -- the energy quantum. Planck's constant, h, was calculated to be about 6.6 x 10-34 Joule second. All this was in opposition to Maxwell's mathematical formulation (and so representation) of the electromagnetic energy as a continuous wave which was then an established part of classical physics. "[Physicists until then] guessed that light was produced by the vibration of electrically charged particles inside an object, vibrations involving the atoms themselves, with the vibrations providing the push needed to set the waves described by Maxwell's equations off and running." (John Gribbin, In Search of the Big Bang, p. 220; c.f. also p. 221-2.) Now Planck had introduced the element of discontinuity or "chunkiness" -- "an element that varies by jumps" ("un élément variant par sauts"), in Marcel Brillouin's words (Rapports, 1912; quoted by editors, Collected Papers of A. Einstein, Vol. 3, Introduction, p. xxvii) -- into the picture of reality, with h "measuring the world's departure from smooth, continuous behavior" of the classical picture of reality, in effect destroying the latter (Cathryn Carson, "The Origin of Quantum Theory", Beamline, summer, 2000.) This new picture was necessary to fit the experimental data; reality as "quantized" explained why we never experienced ultraviolet catastrophe. The distribution of black-body radiation was now given by:
which replaces Jeans-Raleigh's eidos. "Planck's law fits the data perfectly over the whole of the spectrum. In other words, Planck's law reduces to the Raleigh-Jeans law for large wavelengths. In a sense, Planck's law extends classical physics rather than overthrowing it. This is analogous to the way special relativity agrees with classical physics at low speeds." (J. Vacarro, ibid.) The ultraviolet catastrophe was avoided because in the region of high frequency the quantum was so large (e = hf) that only a few vibration modes were excited (Figure below), so that only a few oscillators were actually vibrating. That is, an equipartition of energy was prevented such that we wouldn't be burned dead simply standing in front of fire. 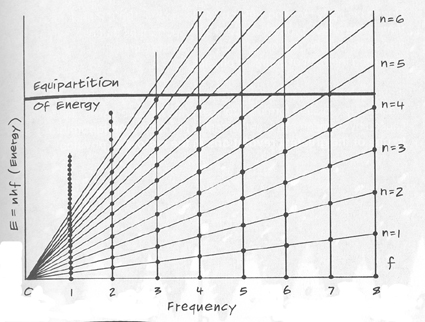
b. The photoelectric effect. Hertz (1887) was the first to notice the so-called "photoelectric effect", that when light fell on certain metals, electrons were ejected from the surface. Light apparently had provided (kinetic) energy for the electrons bound up in (the atoms of) the metal to leave it. The experimental method roughly consisted in illuminating a metal plate called the emitter from which the electrons were ejected and received by another plate installed called the collector. But, as Philip Lenard continued experimentation with the photoelectric effect, however, he noticed details which contradicted the classical picture of light: the "effect" could not have been produced by the "structure" as hitherto envisioned by (classical) mechanics, that light was a continuous wave that carried energy uniformly along its wavefront. The problem was two-fold: (1) "Consider a beam of light of intensity 10-5 Wm-2 falling on an atom of cross-sectional area 10-20m2. The atom receives 10-25W, which is about 5 x 10-7eV per second... Electrons are bound to the metal by a few eV. It would take about 107 seconds, or more than 100 days, for an atom to receive this much energy. But, photoelectrons are found immediately the light is switched on!" (Vacarro.) (2) The (kinetic) energy carried by the electrons knocked off by light brighter or shone closer to the metal remained the same as the energy carried by those knocked off by dimmer light or light shone farther off, provided that it was light of the same wavelength and so same frequency, the difference being that the light of the former case knocked off more electrons. On the other hand if the light was of a different frequency, e.g. of higher frequency (and therefore shorter wavelength), then indeed the electrons knocked off each carried more energy, even if the light was dimmer than the light of lower frequency. It seems that the energy of the light that was the function of its brightness determined the number of electrons knocked off, but that the energy of the light that was the function of its frequency determined the (kinetic) energy level of the individual electrons knocked off. "Also, for frequencies below a certain value, the cut-off frequency, f0, no photoelectrons are produced, regardless of how intense the light is made. However, classical theory predicts that some photoelectrons would be produced at all frequencies." (Vacarro.) The classical picture of light thus had to be wrong, and it was Einstein who attempted to find a new eidos whose effect would fit the newly acquired data but which would give a completely different picture (structure) of light than the classical one, and yet converge with and in fact extend the Planck's new picture in regard to energy. Einstein speculated (1905) that light behaved "as if it were localized in discrete chunks", but he showed this differently than Planck, through the application of thermodynamics to light. (Carson, ibid.) He first resorted to Wien's formula, valid only for the higher frequency spectrum of the black-body radiation (E = af3exp(-bf/T)). He then considered Boltzmann's conception of the fluctuation of a system, that when a system of gas molecules reached maximal entropy the probability emerged that entropy would suddenly decrease with all molecules converging into one corner instead of being evenly distributed throughout space. Einstein found that the behaviour of radiation was very similar to the compressed gas in this case, then applied Boltzmann's entropy formula S = kLogW to the case of radiation, and discovered that within the validity of the Wien law, i.e. within the high-frequency areas, radiation behaved just as if it consisted of mutually independent energy quanta of magnitude kbf (thus, E = nkbf, where the constant b came from Wien). As Planck had already shown that b = h/k, the total energy of the system in terms of E = Nkbf reduced simply to Planck's E = Nhf. In this way Einstein demonstrated that Planck's energy quantum e = hf (= hc/l) not only characterized the "oscillators" in the blackbody but was also the energy quantum characteristic of all radiation: the photon (later so named by Compton), an indivisible, smallest possible chunk of radiation-energy with the magnitude hf, was thus born. Quanta were now understood to be real physical objects (photons), and the energy of light was concentrated in such packets instead of being spread out continuously and uniformly as in the classical picture. This new picture resolved the former paradoxes in that:
Elsewhere Einstein also used the same hf to describe the peculiarity in the energy-change of solids associated with their temperature-change. (Carson, ibid.) In this way quantization, or discontinuity in energy-change, was demonstrated both in force (light) and in matter (solid state). (Stephen Wolfram) Explaining the spectra of hydrogen atoms. This first stage of the origin of quantum theory came to fruition with the quantization of the atomic structure. The issue related to that of "spectra." It had already been widely known that the spectrum of light emitted from the gas being heated of an element consisted of distinct bright lines, and that each element in the gaseous state gave off a peculiar arrangement of such spectra lines that was different than that given off by any other elements, so much so that the spectra lines could be used to identify the gases, such as in the case of the identification of the helium gas in the sun in the 1860s. The spectra lines of an element were thus believed to hold the secrets about the structure of the atom. In this way the physicists attempted to examine the spectra lines of the simplest element, hydrogen, hoping to obtain the physical picture of what the atom looked like. The spectra lines of hydrogen were arranged like this:
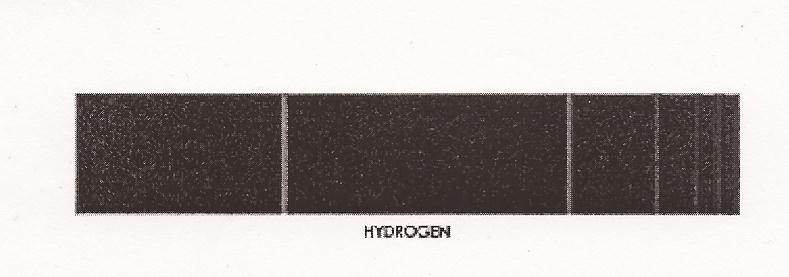 A Swiss school mathematics teacher, Johann Jakob Balmer, tried to find a formula involving whole numbers which would predict exactly the frequencies of the four prominently visible spectra lines of hydrogen; if he could, then he would have discovered the eidos underlying the hydrogen spectra lines. And he did find the formula in 1885:
Where R is the Rydberg constant equal to about 3.29163 x 1015 cycles/sec. The formula was a feat of numerology, not of physics. It could predict the frequencies of the four hydrogen lines (i.e. serve as their eidos) if nf (final) was chosen to be 2 and ni (initial) to be 3, 4, 5, and 6. It could also predict other series of spectra lines in the ultraviolet and infrared regions when different values than 2 were chosen for nf. For example, Lyman discovered (1906 - 14) the series in ultraviolet region when nf was set to 1 and ni to 2, 3, 4, 5... etc.; Paschen discovered (1908) the series in infrared with nf = 3 and ni = 4, 5, 6, 7... etc.; and Brackett discovered (1922) further series in infrared with nf = 4 and ni = 5, 6, 7, 8... etc. (Diagram below.) Clearly, when the atoms of a gas were heated, they underwent a change in energy level, and these frequencies of the light emitted from (or absorbed by) the heated gas, predicted so well by the Balmer formula, must reflect this change of energy. Within the Balmer formula -- within this eidos -- was hidden information about the physical structure of atoms, since the changes of whole numbers involved in the setting of the value of nf, which gave such precise frequencies of the emitted radiation, suggested that some rearrangement of the parts of the atom was taking place during the heating. (McEvoy, ibid. p. 68 - 9.)
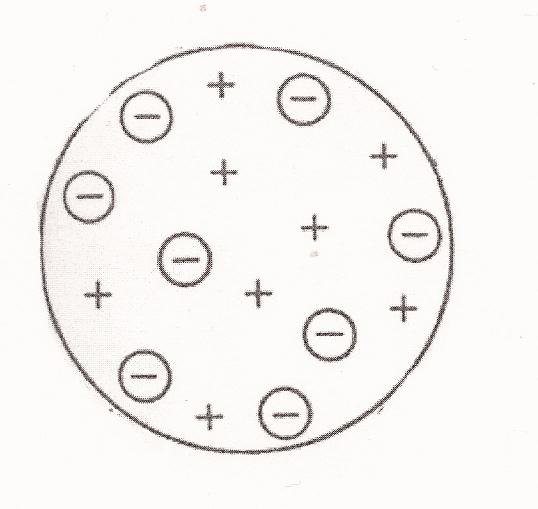
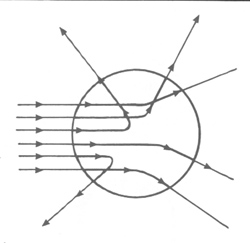
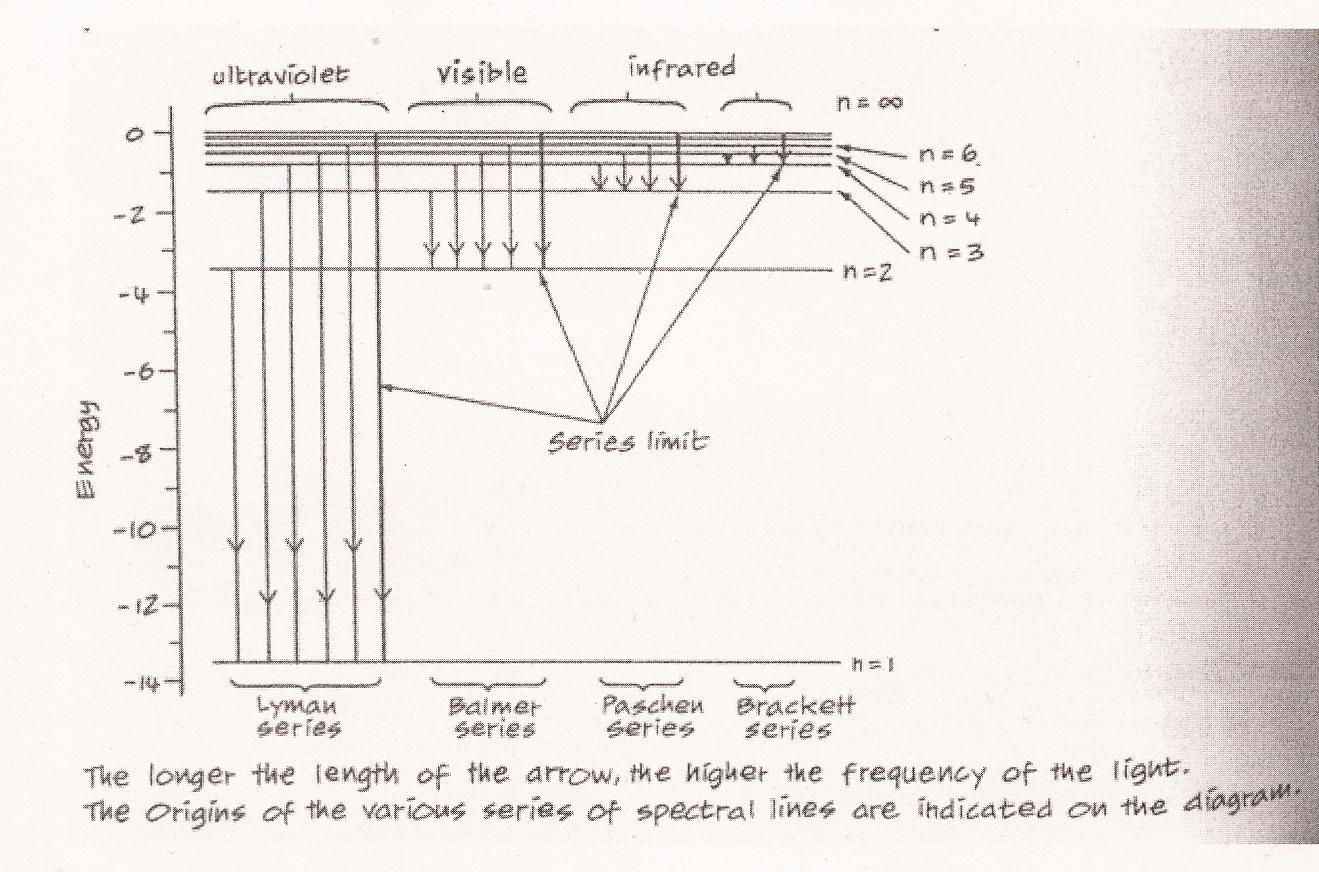 The preliminary visualization of the atom. At the time J. J. Thomson and others had through experiments realized that what had been known as rays (e.g. alpha and beta rays) were actually streams of particles: electrons with negative charge. This is another instance where what appeared on the macroscopic level as continuous were upon closer examination -- on the microscopic level -- actually made of discontinuous "chunks." Thomson and Lord Kelvin then developed the first visualization of the structure of the atom, the "raisins in a pudding" model (Figure below), in which the negatively charged electrons were embedded and distributed evenly in a uniform sphere of positive charge. (Or in Bohr's words, "the atom consists of a sphere of uniform positive electrification, inside which the electrons move in circular orbits"; "On the Constitution of Atoms and Molecules", Philosophical Magazine, Series 6, Vol. 26, July 1913.) They assumed that the two principal constituents of classical mechanics, the electromagnetic theory of Maxwell and Newton's laws of motion, would still hold in the interior of this atom, the former governing the radiation from it and the latter the dynamics within it. Ernest Rutherford with his students Geiger and Marsden however found otherwise with their experiments. By shooting alpha particles on a thin gold foil (alpha scattering), they discovered that while most of them went through the foil completely with only slight deflection, a very minority of them were scattered back completely (Figure below). This showed that the greatest part of the mass of the atom was concentrated in a tiny nucleus in the center, occupying only about one billionth of the space of the atom, causing the backward scattering of those very few alpha particles lucky enough to have hit it, while around the nucleus in the atom there was mostly just empty space, allowing the majority of the particles to pass through. Rutherford thus proposed the preliminary visualization of the structure of the atom, in the manner of celestial mechanics of a positively charged nucleus orbited by negatively charged electrons like the sun orbited by the planets -- with the difference that the planets were held in orbits to the sun by gravity but the electrons to the nucleus by electromagnetism. Now Robert Boyle’s ‘corpuscular’ view of nature, seeing matter as consisting of tiny individual particles, had at last come to its fruition: the first experimentally verified visualization of the structure of the (as yet) indivisible constituent of things existent.
But this atom would be inherently unstable because the application of Maxwell's electromagnetic theory to it indicated that the orbiting motion of the electron would cause it to radiate all its energy away in just a fraction of a second and so to crush into the nucleus. What kept it in orbit continuously? Adopting this planetary model of the atomic structure, Niels Bohr attempted to solve this problem by relating it to Planck-Einstein's quantum relation between the energy of a photon and its frequency, E = hf. For this purpose he made use of the quantized angular momentum which J. W. Nicholson had already produced (!): recall that angular momentum was represented as L = mvr; after quantization, L = mvr = n(h/2p) (h/2p would soon be known as h-cross). Bohr now quantized the orbits of the electron as well, i.e. that electrons could only exist in fixed orbits around the nucleus, "that these orbits were separated by intervals corresponding to a basic quantum of energy and that an electron could jump from one orbit to another but could not exist in an in-between state." (Gribbin, ibid., p. 224) Such quantization of orbits meant that the angular momentum of the electron could only increase or decrease quantum-ly, by some whole number (n) times h-cross. Hence L = mvr = n(h/2p) for the first, nearest orbit around the nucleus, the ground state of the atom (known as the eigenstate, the state in which an unexcited hydrogen atom would have its electron), would be L = 1(h/2p), and for the second orbit, L = 2(h/2p), etc. The integer n can be 1, 2, 3, 17, 108, and so on. Bohr removed the problem of the instability of the atom by postulating -- this is his first postulate -- each fixed orbit as "stationary", meaning that the electron in it was orbiting without the emission of radiation, the orbit characterized only by the condition L = mvr = n(h/2p). Having already referred to "the inadequacy of the classical electrodynamics in describing the behaviour of systems of atomic size" ("On the Constitution ..."), he thus threw out the classical electromagnetic theory altogether. (Carson, ibid.) Bohr could also calculate (represent) the radii (r) of the electronic orbits using the classical, Newtonian manner for the solar system:
where e is the charge of the electron and m its mass. We see here that since the time of Boyle and Lavoisier when materiality, which was until then common-sensely (i.e. macroscopically) regarded as continuous, and which by then comprehended three physical states of gaseousness, liquidity, and solidity, was first made "chunky", the composite of indivisible, discontinuous constituents (molecules), now first energy, and then even motion and orbit -- because the latter two depend on the former -- which classical mechanics took to be continuous, in accordance with common sense on the macroscopic level, turned out to be chunky as well: the meaning of "quantization." Bohr's second postulate was that the jump of the electron from higher energy orbit to a lower one caused the electron to emit a photon of energy hf -- whence the observed bright lines of spectra -- and the jump from lower to higher would require the absorption of a photon of energy hf -- whence the observed dark lines or "gaps" in the spectrum -- both in accordance with Planck's and Einstein's quantization imposed on the continuity of the classical picture. The energy emitted when the electron jumped to a lower energy orbit could then be calculated as hf = Ei - Ef (Ei = the energy level of the initial orbit, Ef = that of the final orbit). Let's look into the details of Bohr's articulation of this. The energy E of the (hydrogen) atomic system (for the particular orbital state of its electron) Bohr defined as equal to "the mean value of the kinetic energy of the electron taken for a whole revolution" (Bohr, ibid., p. 3). He furthermore defined the frequency of the energy emitted as equal to half of revolution (w). ("... the frequency of the radiation emitted during the passing of the system from a state in which no energy is yet radiated out to one of the stationary states, is equal to half the frequency of revolution of the electron in the latter state", p. 8) In this way, with Q designating the charge of the nucleus,
Since the charge of the electron e was of the same strength as that of the nucleus. This meant that "The amount of energy emitted by the passing of the system from a state corresponding to [ni] to one corresponding to [nf] is consequently" (p. 8):
Since f = h(w/2), Bohr could then derive Balmer's formula (!):
If, that is, the Rydberg constant R = (2p2me4)/h3. Bohr calculated this latter to be 3.1 x 1015, compared with the former value 3.29163 x 1015: "The agreement between the theoretical and observed values is inside the uncertainty due to experimental errors in the constants entering in the expression for the theoretical value." (Ibid., p. 9) Hence he considered himself having succeeded. All these eidoi must be mutually derivable one from another because they are all surface effects of some more fundamental eidos (structure). Thus with the derivation in place, the picture of the physical structure of the atom underlying the surface effects of spectra lines can be drawn:
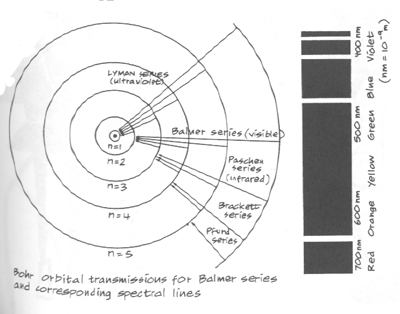 (From McEvoy) "We see that [our equation] accounts for the law connecting lines in the spectrum of hydrogen. If we put [nf] = 2 and let [ni] vary, we get the ordinary Balmer series. If we put [nf] = 3, we get the series in the ultra-red observed by Paschen and previously suspected by Ritz. If we put [nf] = 1 and [nf] = 4, 5, . . , we get series respectively in the extreme ultra-violet and the extreme ultra-red, which are not observed, but the existence of which may be expected." (Ibid., p. 9) This demonstrates that the one dimensional representation, or eidos, which Bohr had isolated for the frequency of the energy emitted, since it was the "right one", underlay, and would underlie, all the other spectra lines many had observed in the light here or from celestial bodies in other regions of the spectrum and for other elements. Another example Bohr gave: "It will be observed that we in the above way do not obtain other series of lines, generally ascribed to hydrogen; for instance, the series first observed by Pickering in the spectrum of the star z Puppis, and the set of series recently found by Fowler by experiments with vacuum tubes containing a mixture of hydrogen and helium. We shall, however, see that, by help of the above theory, we can account naturally for these series of lines if we ascribe them to helium... A neutral atom of [this] element consists, according to Rutherford's theory, of a positive nucleus of charge 2e and two electrons. Now considering the binding of a single electron by a helium nucleus, we get, putting E = 2e... and proceeding in exactly the same way as above,
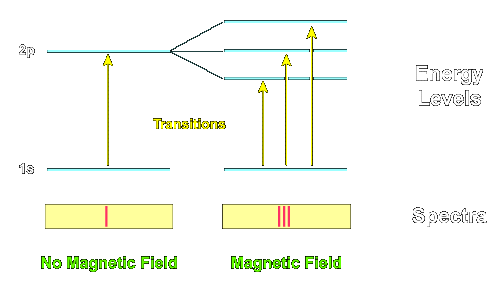
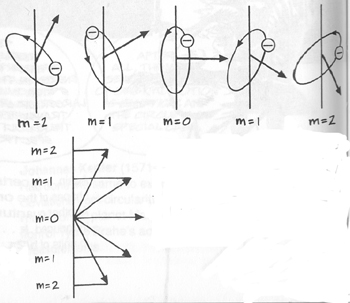
If we in this formula put [nf] = 1 or [nf] = 2, we get series of lines in the extreme ultra-violet. If we put [nf] = 3, and let [ni] vary, we get a series which includes 2 of the series observed by Fowler, and denoted by him as the first and second principal series of the hydrogen spectrum. If we put [nf] = 4, we get the series observed by Pickering in the spectrum of z Puppis. Every second of the lines in this series is identical with a line in the Balmer series of the hydrogen spectrum; the presence of hydrogen in the star in question may therefore account for the fact that these lines are of a greater intensity than the rest of the lines in the series. The series is also observed in the experiments of Fowler, and denoted in his paper as the Sharp series of the hydrogen spectrum. If we finally in the above formula put [nf] = 5, 6, . . , we get a series the strong lines of which are to be expected in the ultra-red." (Ibid., p. 10) Again, the predictability as seen here means jus that Bohr's eidos for frequency has succeeded in capturing the structure underneath the functions of spectra lines, and this structure, representation, of frequency is finally grounded in the completed version of Rutherford's model for the atomic structure, as visualized above. Bohr's eidos turned out to be just part of a much complicated picture, however, as more spectra lines appeared, even in the simple hydrogen. It was apparently incomplete. There were apparently more possible states for the electron than Bohr's circular orbits (designated with one quantum number n) allowed. Arnold Sommerfeld solved the problem by extending Bohr's circular orbits into elliptical ones, which was natural since, as seen, the circle is just a special case of ellipse (ellipse with zero eccentricity). Thus Kepler's discovery in the case of the planetary orbits that the circular orbit of constant velocity was simply a partial manifestation of the elliptical orbit of "equal area in equal time" was now extended to electronic orbits. But again, only certain values of the shapes of the orbit were allowed -- the ellipse itself was quantized in its changing eccentricity --, designated by the additional constant k. Then Pieter Zeeman discovered that even more spectra lines appeared when the excited atoms were placed in a magnetic field: the Zeeman effect. Apparently, the direction or orientation of the electronic orbit had also to be taken into account, as, when the magnetic field was applied, the excited electron could select from various orbits pointing in various directions in relation to the field, or, to put it another way, the field affected the precession of the orbital angular momentum vector with respect to it, similar to the precession of the axis of a spinning top in a gravitational field -- this allowing different energies. Another constant m now had to be introduced, since these directions were also quantized (!). The transitions in energy state of the atom were now calculated on the basis of three quantum numbers: the size of the orbit (n), the shape of the orbit (k), and the direction in which the orbit was pointing (m). (McEvoy)
(From "Zeeman Effect", Uni. of Tennessee)
Take note that the equation which was supposed to capture the energy-shift caused by the changing precession of the orbital angular momentum effected by the magnetic field:
(where mB [e(h-cross)/2me] is named the Bohr magneton; reference) was the eidos that corresponded to the visualization of this changing precession above. The Anomalous Zeeman Effect and the electronic spin But later magnetic field produced more spectral lines. These became known as the Anomalous Zeeman Effect. Apparently another feature of the orbiting electron must have been disturbed which was not yet known. Uhlenbeck and Goudsmit (with their professor Ehrenfest) at Leyden thought that perhaps the electron was also spinning while orbiting just as the earth did on its axis when orbiting around the sun and that it was the extra angular momentum given by this spinning which was disturbed. What was called the Anomalous Zeeman Effect really was just the normal Zeeman Effect if electron’s spin was taken into account. Hence one merely added the spin angular momentum (multiplied by 2) into the previous equation to get the new eidos capturing the energy-shift in the Anomalous Zeeman Effect (ibid.):
(Reference, ibid.) At this point Wolfgang Pauli, after being convinced of this, postulated that the direction of the rotation of the spinning of the electron could either be clockwise (spin-up) or counter-clockwise (spin-down). But then the angular momentum of the spinning electron turned out to be only one-half of the normal value h/2p of atomic orbits, that is, spin 1/2. This created problem for the visualization of the electronic movement, as it became somewhat un-visualizable, since this meant that the electron would have to spin around twice to get back to its original starting point. The problem was to intensify further later on when electron was demonstrated to be wave-like (below), and how did wave spin? Until Bohr finally established, in 1932, “that electron spin cannot be measured by any classical experiment, such as deflection of beams of electrons by magnetic fields. It is a property that only appears in quantum interactions, such as the ones that produce the splitting of the spectral lines, and it has no classical meaning whatsoever.” (Gribbin, The Search for Schrödinger’s Cat, p. 94.) This problem of “picturization” (Anschaulichkeit, in the words of Schrödinger) at the atomic level would later become such a major problem in quantum mechanics. The physical structure underlying the periodicity of the Periodic Table. So, then, the purpose of all this was to provide the physical picture of the structure of the atom that underlay the spectral emission which was thus the effect (function) of this structure. Now that this picture was more or less “complete” (electronic spin notwithstanding), Bohr could also use it to show how the periodicity of the elements organized in the Periodic Table could arise as the surface effect of the physical structure of these elements – by furnishing, as he summarily explained in his letter to Nature, "Atomic Structure" (March 24, 1921), “a detailed picture of the constitution and formation of [the] groups [of electrons inside these elements].” For now, Bohr “recognized that the various physical and chemical properties of the elements depend on the electrons moving around the nuclei of their atoms and that only the atomic weight and possible radioactive behavior are determined by the small but massive nucleus itself.” (Martin J. Klein, "Niels Bohr in Britannica Nobel Prizes)
Throughout his attempt to utilize quantum theory to “theorize” about (in the original sense: look at) the atomic structure Bohr was guided by his “correspondence principle”: “According to this principle, every transition process between stationary states as given by the quantum postulate can be ‘coordinated’ with a corresponding harmonic component (of a single frequency) in the motion of the electrons as described by classical mechanics.” (Klein)
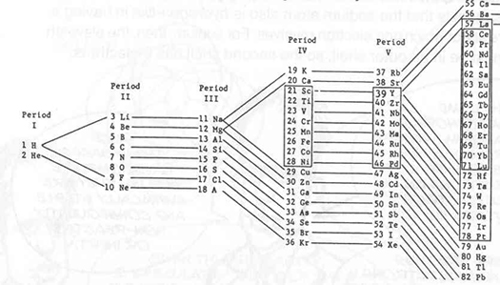
Thus, a fancy numerology could be built up: orbit 1: 2 x 1 = 2; orbit 2: 4 x 2 = 8; orbit 3: 6 x 3 = 18; orbit 4: 8 x 4 = 32.
On the opposite side of inert gas (group 18), elements of group 1 (from period 1 to 6: Hydrogen with atomic number 1, Lithium (Li), 3, Sodium (Na), 11, Potassium (K), 19, Rubidium (Rb), 37, and Caesium (Cs), 55) all have similar chemical properties or reactivity because they all have just one electron on their outermost electronic orbit, which was expected to be easily involved with (to combine with the outlying electrons of) neighboring atoms; thus the reactivity of this group was “explained.”
By carrying out an analysis of all the elements along this line, Bohr in 1921 re-structured the periodic table in the form shown below. Remember that this was supposed to be the governing principle for, i.e. the structure underlying, the formation of the elemental constituents of the geosphere.
 The Pauli Exclusion Principle But this structure of the electronic shells, explaining well the periodicity of elements though it might be, itself lacked a reason. As “Bohr talked of the electrons as being in ‘shells’ around the nucleus, with ‘new’ electrons going into the shell with the least energy until it was full, and then into the next shell, and so on… he did not explain how or why a shell became full – why the first shell could contain only two electrons, but the next eight, and so on” (Gribbin, ibid., p. 96), that fancy numerology lacking a foundation. “Each of Bohr’s shells corresponded to a set of quantum numbers, and Pauli realized in 1925 that with the addition of his fourth quantum number for the electron the number of electrons in each full shell exactly corresponded to the number of different sets of quantum numbers belonging to that shell” (ibid.). That is, his formulation of the electronic spin into what is now known as the Pauli Exclusion Principle provided the needed structural principle for the formation of electronic shells, and so for the periodicity of the elements of the geosphere (or rather of the matter aspect of it). This principle stated that each atomic state defined by the previous three quantum numbers contained two electrons and needed its exclusive orbit, or reversely that no two electrons could have the same set of quantum numbers, or namely that each quantum state, each distinct energy level, was characterized by not three but four quantum numbers, counting spin up or down.
The fourth shell would then have 2 + 6 + 10 + 14 = 32 electronic states, which meant 32 electrons. The mathematical equation that would capture this table would then be the eidos for the structure of the electronic shells, and so for the chemical properties of the elements of the geosphere. “Because of the almost headlong progress in physics in 1925 and 1926, the importance of exclusion sometimes gets overlooked, but it is, in fact, a concept as fundamental and far reaching as the concept of relativity. The Pauli exclusion principle, it turns out, applies to all particles that have a half-integer amount of spin – 1/2( h/2p), 3/2(h/2p), 5/2(h/2p) and so on. Particles that have no spin at all (like photons) or integer spin (h/2p, 2 h/2p , 3 h/2p and so on) behave in a completely different fashion, following a different set of rules. The rules that are obeyed by the half-spin particles are called Fermi-Dirac statistics, after Enrico Fermi and Paul Dirac, who worked them out in 1925 and 1926. Such particles are called ‘fermions.’ The rules obeyed by full-spin particles are called Bose-Einstein statistics, after the two men who worked them out, and the particles are called ‘bosons.’” (Gribbin, ibid., p. 97.) Fermions were the constituents of matter and bosons, the carriers of force. The property of matter was entirely due to the Pauli Exclusion Principle, which prevented atoms from always collapsing to its lowest or ground state and electrons from getting on top of each other, thus ensuring that one piece of matter could not go through another, as we cannot go through walls. Force particles, on the other hand, could subsist through matter and thus helped pieces of matter interact with one another. We need only space-time (as the context of matter and interaction within matter) to complete the entire picture of the geosphere. Electron made into wave. De Broglie, at Sorbonne, in 1923, thought that if it was possible for electromagnetism -- which had been "classically" established as wave by Maxwell -- to appear as stream of particles (photons), then it might be possible for what was established as particle (e.g. electron) to appear as wave. He reformulated the electron as wave via a seemingly mathematical slight of hand. So far, the energy of a particle (matter) was E = mc2, and the energy of force (e.g. photon) was E = hn. Momentum (p) was mass x velocity (p = mv). Since the velocity of the photon was c (speed of light), its momentum was p = mc, and its energy was E = mcc = mvc = pc, this formula applicable to both electron (matter) and to photon3. Therefore E = pc and pc = hn, and so p (momentum) = hn/c; but velocity (c)/ frequency (n) = wavelength l, so that p = h/l. (If p = hn/c, then h = p/(n/c) = p (c/n), and so p = h/(c/n) or h/l.) After that, h = pl and l = h/p, that is, wavelength = Planck's constant (h) divided by momentum (p), so that every particle was also wave, and its wavelength depended on Planck's constant and its momentum. (Gribbin, ibid., p. 225-7) Although De Broglie's presentation of the wavelength of the electron was not well received at the time, by 1929, when he was awarded the Nobel prize for this, it was well accepted that waves had to be treated like particles and particles waves. For ordinary objects, mass (and so momentum) is so huge that h/p is too small, so that their wave function is not noticeable. Only with electron, with the small mass of 9 x 10-28 gram, is its wave-ness apparent (Gribbin, ibid.). Again our ordinary experience of stable solid things moving in determinate and definite course -- exactly, i.e. quantitatively represented by the classical Newtonian physics -- is gradually being revealed by the structural perspective of science to be illusory, a partial picture of the whole, in this case the stable solidity being the way reality appears only on the specific macroscopic level (of large mass) proper to our existence and in slow speed. With Bohr's atomic model, the first, or "old" quantum theory had come to fruition. After De Broglie, nature was revealed to be ever stranger, i.e. no longer Newtonian-Maxwellian, and a process began of "a full-scale replacement of classical physics." (Carson, ibid.) Quantum mechanics "Discontinuity, abstraction from the visualizable, a positivistic turn towards observable quantities [i.e. dropping the ideal of picturization due to its somewhat impossibility]; these preferences indicated one path to a full quantum theory. So however, did their opposites. When in 1925-1926 a true quantum mechanics was finally achieved, two seemingly distinct alternatives were on the offer." (Carson, ibid.) What is referred to here is the opposition between Heisenberg's matrix mechanics and Schroedinger's wave mechanics, somewhat an opposition between the functional and the structural, and one founded on Planck's constant (discontinuity) and the other on De Broglie's electronic wave. The break-through paper of 1925 that established Heisenberg's matrix mechanics is "On the Quantum-Theoretical Reinterpretation of Kinematical and Mechanical Relations" ("Ueber quantentheoretische Umdeutung kinematischer und mechanischer Beziehungen" in Zeitschrift für Physik, 33 (1925), 879-893). "Since the electron orbits in atoms could not be observed [since, ultimately, as the case of electronic spin had already demonstrated, 'electron orbit and spin' escaped all classical, common-sense visualization which was based in the macroscopic world], Heisenberg tried to develop a quantum mechanics without them. He relied instead on what can be observed, namely the light emitted and absorbed by the atoms." (American Institute of Physics, exhibit on Heisenberg) That is, "frequencies and amplitudes associated with the line intensities" were to be the object of mathematical representation (description), not the electronic orbits whose dynamics presumably produced these. (Quote from Heisenberg, Physics and Beyond, p. 60.) This is then pretty much a functional approach, or regression thereto, dealing with the effects (line spectra representing the emission and absorption of light) rather than with the structure (the change of orbit by the electron which had either emitted or absorbed light), and in this way laying out the path toward the later bootstrap approach which similarly does not deal with quarks that cannot be observed but only with their effects, the transformations of hadronic structures from one into the other. The focus on the functions, on the effects, of the electronic orbits rather than on the orbits themselves was what Heisenberg insisted on as the "observable quantities" ("beobachtbare Groessen"). "The present paper seeks to establish a basis for theoretical quantum mechanics founded exclusively upon relationships between quantities which in principle are observable", thus so begins Heisenberg his paper. ("In der Arbeit soll versucht werden, Grundlagen zu gewinnen fuer eine quantentheoretische Mechanik, die ausschliesslich auf Beziehungen zwischen prinzipiell beobachtbare Groessen basiert ist." Ibid.)4
Footnotes:
1. J. P. McEvoy's Introducing Quantum Theory (1996; Icon Books, UK, 2004), though comic, presents a very good, fairly detailed account of the origin of quantum theory. The following narrative incorporates many elements from this book.
2. The frequency of radiation, also designated by the Greek letter nu, n, is measured as the number of wave-crests passing by a fixed point every second. Light, that visible portion of electromagnetism, has a wavelength of 10-7m (meter) and a velocity of 300,000 km/sec. Its frequency is therefore around 3 x 1015 per second (or Hertz).
3. A paradox may be involved here, because the mass of photon is zero, so the energy of photon E = mcc seems to always be zero. But the original equation of relativity of Einstein establishing the equivalence between mass and energy is actually E2 = m2c4 + p2c2 where p is momentum, shortened to E = mc2 when momentum is zero. E = mc2 denotes therefore only rest energy of a particle, which, if moving, must also have additional E = pc. For photon, its entire energy lies in the product of its momentum times its velocity, pc.
4. Other problems enumerated on the front page are: "dass sich nur das Wasserstoffatom und der starkeffekt dieses Atoms jenen formalen Regeln der Quantentheorie fuegen"; "dass die Reaktion der Atome auf periodisch welchsende [elektrischen und magnetischen] Felder sicherlich nicht durch die genannten Regeln beschrieben werden kann, und dass schliesslich eine Ausdehnung der Quantenregeln auf die Behandlung der Atome mit mehreren Elektronen sich als unmoeglich erwiesen hat." ("That only hydrogen atom and its intensity effect are describable by the formal laws of quantum theory [known so far]"; "that the reaction of atoms in the periodically changing [electromagnetic] field cannot be described by the quantum laws just mentioned, and that an extension of the quantum laws to the handling of atoms with more electrons [than hydrogen] has [thus far] been shown strictly impossible".)
|

 The appearance of photon: a. The quantum. Whereas all objects absorb and emit electromagnetic radiation (usually in the infrared part of the spectrum) from and to their surroundings, a black body is one that absorbs all available energy and emits it all (hence the electromagnetic energy coming from it is not due to reflection). For example, a hollow cavity with a small hole in it can serve as an approximation of a black body (left: from J. Vacarro's lecture, "
The appearance of photon: a. The quantum. Whereas all objects absorb and emit electromagnetic radiation (usually in the infrared part of the spectrum) from and to their surroundings, a black body is one that absorbs all available energy and emits it all (hence the electromagnetic energy coming from it is not due to reflection). For example, a hollow cavity with a small hole in it can serve as an approximation of a black body (left: from J. Vacarro's lecture, "